Unit B review - mvhs
... 2nd IE is removing an e- from a filled shell in Na and K, but since the e- is being removed from a shell closer to the nucleus in Na, it is larger. A most inner e- shells, most shielding C EN increases as you go up a group E removing an e- from a half-filled p subshell in N takes more energy A All a ...
... 2nd IE is removing an e- from a filled shell in Na and K, but since the e- is being removed from a shell closer to the nucleus in Na, it is larger. A most inner e- shells, most shielding C EN increases as you go up a group E removing an e- from a half-filled p subshell in N takes more energy A All a ...
document
... particle as it moves through the gas collect at the wires. Their arrival at a particular point on the wire is recorded as a current. The electrons or ions take a certain time to drift to the nearest wire. This time is recorded and used to calculate the precise location where the electron or ion was ...
... particle as it moves through the gas collect at the wires. Their arrival at a particular point on the wire is recorded as a current. The electrons or ions take a certain time to drift to the nearest wire. This time is recorded and used to calculate the precise location where the electron or ion was ...
Introduction to Electromagnetism
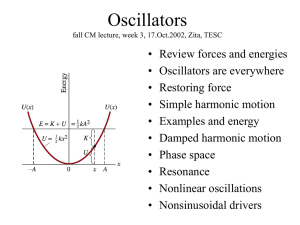
... Second-order diffeq needs two linearly independent solutions: x = x1 + x2. Unknown coefficients to be determined by BC. Sub in your solution and solve for angular frequency w 2 f ...
... Second-order diffeq needs two linearly independent solutions: x = x1 + x2. Unknown coefficients to be determined by BC. Sub in your solution and solve for angular frequency w 2 f ...
Using facets as a tool to interpret a survey on quantization
... •There is a mean radial position that can be defined for each energy level. •There is a characteristic shape for each energy level. •There is a ground state energy level characterized by having the lowest possible energy. •“Higher” energy levels have energies between the ground state energy and zero ...
... •There is a mean radial position that can be defined for each energy level. •There is a characteristic shape for each energy level. •There is a ground state energy level characterized by having the lowest possible energy. •“Higher” energy levels have energies between the ground state energy and zero ...
Unit 3 Lesson 3: Electromagnetic Spectrum
... Fire in the Sky • The stream of electrically charged particles from the sun is called the solar wind. • When solar wind encounters Earth’s magnetic field, the particles are accelerated. • When the accelerated particles collide with the atmosphere, they give off EM radiation in the form of light. ...
... Fire in the Sky • The stream of electrically charged particles from the sun is called the solar wind. • When solar wind encounters Earth’s magnetic field, the particles are accelerated. • When the accelerated particles collide with the atmosphere, they give off EM radiation in the form of light. ...
electron configuration
... Filling Patterns of Atomic Orbitals • Atomic Orbitals are filled from lowest energy to highest energy • Full/half full atomic orbitals – Adding electrons until all orbitals are full is a lower energy state (usually) – Paired electrons are higher energy than unpaired (Hund’s Rule) – Half full orbita ...
... Filling Patterns of Atomic Orbitals • Atomic Orbitals are filled from lowest energy to highest energy • Full/half full atomic orbitals – Adding electrons until all orbitals are full is a lower energy state (usually) – Paired electrons are higher energy than unpaired (Hund’s Rule) – Half full orbita ...
Ray Optics: Reflection and Refraction
... – Apparently causes light to diverge from a mirror or lens. It cannot be seen on a screen or piece of paper since no light actually converges at the image location. ...
... – Apparently causes light to diverge from a mirror or lens. It cannot be seen on a screen or piece of paper since no light actually converges at the image location. ...
Exam Results - University of Wisconsin–Madison
... Quantum Electrodynamics: QED • QED is the relativistic quantum theory of electrons and photons, easily generalized to include other charged particles. • to photon emission or absorption which may be represented by a simple diagram - a Feynman studied the idea that all QED ...
... Quantum Electrodynamics: QED • QED is the relativistic quantum theory of electrons and photons, easily generalized to include other charged particles. • to photon emission or absorption which may be represented by a simple diagram - a Feynman studied the idea that all QED ...
Lecture 11
... Final products have less mass, but much more kinetic energy. Conversion of mass to kinetic energy. As an experimental result: These quantities (momentum and energy as above) ARE conserved. Seen over and over again in high energy experiments. ...
... Final products have less mass, but much more kinetic energy. Conversion of mass to kinetic energy. As an experimental result: These quantities (momentum and energy as above) ARE conserved. Seen over and over again in high energy experiments. ...
Basic Atomic Theory
... • Strength of Coulomb forces much larger than gravitational • +ve and –ve charges cause attractive and repulsive interactions. ...
... • Strength of Coulomb forces much larger than gravitational • +ve and –ve charges cause attractive and repulsive interactions. ...