* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Unit 3 Lesson 3: Electromagnetic Spectrum
Survey
Document related concepts
Transcript
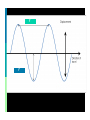
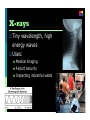
Electromagnetic Spectrum Unit 3 Lesson 3 Unit 3 Lesson 3 The Electromagnetic Spectrum Florida Benchmark • SC.7.P.10.1 Illustrate that the sun’s energy arrives as radiation with a wide range of wavelengths, including infrared, visible, and ultraviolet, and that white light is made up of a spectrum of many different colors. Copyright © Houghton Mifflin Harcourt Publishing Company Waves… a review Most waves are either longitudinal or transverse. Sound waves are longitudinal. But all electromagnetic waves are transverse… ? ? Unit 3 Lesson 3 The Electromagnetic Spectrum Electromagnetic Light Show What is the nature of light? • Light waves are different from other kinds of waves. • When an electrically charged particle vibrates, its fields also vibrate, producing an electromagnetic (EM) wave. • Light waves are vibrating electric and magnetic fields that transfer energy through space. Copyright © Houghton Mifflin Harcourt Publishing Company Electromagnetic waves Produced by the movement of electrically charged particles Can travel in a “vacuum” (they do NOT need a medium Travel at the speed of light Also known as EM waves Wave-particle Duality Light can behave like a wave or like a particle A “particle” of light is called a photon Unit 3 Lesson 3 The Electromagnetic Spectrum What is the nature of light? • EM waves travel as perpendicular electric and magnetic fields. Copyright © Houghton Mifflin Harcourt Publishing Company Unit 3 Lesson 3 The Electromagnetic Spectrum What is the nature of light? • Radiation is energy that has been transmitted by waves or particles. This transfer of energy is called EM radiation. • All EM waves move at the same speed in a vacuum: the speed of light. • EM waves can travel through many materials. Copyright © Houghton Mifflin Harcourt Publishing Company Unit 3 Lesson 3 The Electromagnetic Spectrum What determines the color of light? • Different wavelengths of light are perceived by our eyes as different colors. • White light is what we perceive when we see all the wavelengths of light at once, in equal proportions. • Our eyes only register three colors of light: red, green, and blue. All other colors we see are a mixture of these three colors. Copyright © Houghton Mifflin Harcourt Publishing Company Unit 3 Lesson 3 The Electromagnetic Spectrum Invisible Colors What are the parts of the EM spectrum? • The range of frequencies that EM waves can have are called the electromagnetic (EM) spectrum. Copyright © Houghton Mifflin Harcourt Publishing Company Radio waves Longest wavelength EM waves Uses: TV broadcasting AM and FM broadcast radio Avalanche beacons Heart rate monitors Cell phone communication Microwaves Wavelengths from 1 mm- 1 m Uses: Microwave ovens Bluetooth headsets Broadband Wireless Internet Radar GPS Infrared Radiation Wavelengths in between microwaves and visible light Uses: Night vision goggles Remote controls Heat-seeking missiles Visible light Only type of EM wave able to be detected by the human eye Violet is the highest frequency light Red light is the lowest frequency light Ultraviolet Shorter light Uses: wavelengths than visible Black lights Sterilizing medical equipment Water disinfection Security images on money Ultraviolet (cont.) UVA UVB and UVC Energy Highest of UV waves Lower than UVA Health risks Extremely low risk for DNA damage Can destroy Vitamin A in skin Can cause DNA damage, leading to skin cancer Responsible for sunburn X-rays Tiny wavelength, high energy waves Uses: Medical imaging Airport security Inspecting industrial welds Gamma Rays Smallest wavelengths, highest energy EM waves Uses Food irradiation Cancer treatment Treating wood flooring Unit 3 Lesson 3 The Electromagnetic Spectrum Star Light, Star Bright How much of the sun’s energy reaches us? • Most of the sun’s energy is in the narrow visible light range, but the sun gives off some radiation in every part of the spectrum. Copyright © Houghton Mifflin Harcourt Publishing Company Unit 3 Lesson 3 The Electromagnetic Spectrum How much of the sun’s energy reaches us? • Not all wavelengths of light penetrate the atmosphere equally. Radio waves penetrate the atmosphere easily. Copyright © Houghton Mifflin Harcourt Publishing Company Unit 3 Lesson 3 The Electromagnetic Spectrum How much of the sun’s energy reaches us? • Some EM radiation can be dangerous to humans, so we take extra steps to protect ourselves. • UV light can be harmful. It can penetrate clouds. • In space, the dangers from EM radiation are very high because there is no atmosphere to filter the radiation. Copyright © Houghton Mifflin Harcourt Publishing Company Unit 3 Lesson 3 The Electromagnetic Spectrum Frequency Asked Questions How much energy does EM radiation have? • Different frequencies of EM waves carry different amounts of energy. • High-frequency EM waves have more energy than low-frequency EM waves. Copyright © Houghton Mifflin Harcourt Publishing Company Unit 3 Lesson 3 The Electromagnetic Spectrum How much energy does EM radiation have? • Because low-frequency waves, such as radio waves, carry less energy, they are safer. Walkietalkies and baby monitors use radio waves. • High-frequency waves, such as UV light, carry more energy and can be harmful. UV light causes sunburns, and X-rays require precautions. Copyright © Houghton Mifflin Harcourt Publishing Company Unit 3 Lesson 3 The Electromagnetic Spectrum Fire in the Sky • The stream of electrically charged particles from the sun is called the solar wind. • When solar wind encounters Earth’s magnetic field, the particles are accelerated. • When the accelerated particles collide with the atmosphere, they give off EM radiation in the form of light. Copyright © Houghton Mifflin Harcourt Publishing Company Unit 3 Lesson 3 The Electromagnetic Spectrum Fire in the Sky • Near the poles, the accelerated particles form an aurora that can light up the sky. • The aurora at the North Pole is called the aurora borealis. At the South Pole, it is called the aurora australis. • The color of the aurora depends on the type of atoms in the atmosphere that react with the solar wind. Copyright © Houghton Mifflin Harcourt Publishing Company