enzymes - MrsGorukhomework
... Denatured – structural change so therefore the active site would not be a fit. • ph – have an optimum pH, are sensitive to change, sites get denatured at wrong pH (compartmentalization) • [enzyme] – as [enzyme] increases, so does reaction rate up to a certain point then stabilizes • [substrate] – as ...
... Denatured – structural change so therefore the active site would not be a fit. • ph – have an optimum pH, are sensitive to change, sites get denatured at wrong pH (compartmentalization) • [enzyme] – as [enzyme] increases, so does reaction rate up to a certain point then stabilizes • [substrate] – as ...
Is the decision based on simple thermodynamics?
... exclude water molecules from the volumes they occupy, and they present regions of space where hydrogen bonding cannot occur Chandler, Nature (2005) 437:640-647 ...
... exclude water molecules from the volumes they occupy, and they present regions of space where hydrogen bonding cannot occur Chandler, Nature (2005) 437:640-647 ...
Chemical digestion
... Absorption of glucose and amino acids occurs at the villi (finger like projections). Absorbed by blood. Absorption of fats occurs at the villi; absorbed into lymph system. ...
... Absorption of glucose and amino acids occurs at the villi (finger like projections). Absorbed by blood. Absorption of fats occurs at the villi; absorbed into lymph system. ...
Enzymes -2.Properties, claasification and theories of action (1)
... • Enzymes are highly specific and interact with specific substrates with specific functional groups • Other substrates would not fit into their active sites • It catalyzes only one type of chemical reaction • The set of enzymes present in a cell determines which type of reaction will occur in that c ...
... • Enzymes are highly specific and interact with specific substrates with specific functional groups • Other substrates would not fit into their active sites • It catalyzes only one type of chemical reaction • The set of enzymes present in a cell determines which type of reaction will occur in that c ...
Energy
... • Glucose gives up energy as it is oxidized oxidation = loss of H Oxygen is reduced (gains H) Loss of hydrogen atoms ...
... • Glucose gives up energy as it is oxidized oxidation = loss of H Oxygen is reduced (gains H) Loss of hydrogen atoms ...
Plasma Membrane
... Gases readily diffuse through lipid bilayer. (Ex. movement of oxygen inside cells and CO2 outside) ...
... Gases readily diffuse through lipid bilayer. (Ex. movement of oxygen inside cells and CO2 outside) ...
Practice Exam II answers
... (b), post-translational modification/hydroxylation of proline and lysine residues allows for semi-aldehyde formation between individual collagen helices. 12). What result on Hb would you expect from a random mutation at the 12-interface, which resulted in the creation of an extra ionic bond betwe ...
... (b), post-translational modification/hydroxylation of proline and lysine residues allows for semi-aldehyde formation between individual collagen helices. 12). What result on Hb would you expect from a random mutation at the 12-interface, which resulted in the creation of an extra ionic bond betwe ...
Cell Membrane Information
... The cell membrane is also called the plasma membrane. It is made of a phospholipid bilayer. This double layer of lipids is also known as a fluid mosaic because the phospholipids that make up the membrane lie next to each other but are not connected. This gives the cell membrane a fluid motion. The c ...
... The cell membrane is also called the plasma membrane. It is made of a phospholipid bilayer. This double layer of lipids is also known as a fluid mosaic because the phospholipids that make up the membrane lie next to each other but are not connected. This gives the cell membrane a fluid motion. The c ...
GLYCOLYSIS Generation of ATP from Metabolic Fuels
... CHM333 LECTURE 27 & 28: 4/2 – 4/4/2012 Spring 2012 ...
... CHM333 LECTURE 27 & 28: 4/2 – 4/4/2012 Spring 2012 ...
to find the lecture notes for lecture 1 click here
... energy to cause their chemical bonds to become unstable and created new ones – as these bonds form – energy is released into the environment – if more energy is released than absorbed = heat (exothermic reaction) – two influences on AE – temperature and concentration • concentration – increasing thi ...
... energy to cause their chemical bonds to become unstable and created new ones – as these bonds form – energy is released into the environment – if more energy is released than absorbed = heat (exothermic reaction) – two influences on AE – temperature and concentration • concentration – increasing thi ...
Bioenergetics
... not the mitochondria • For intense exercise of 1 to 2 minutes (e.g., jumping rope) duration, glycolysis provides the primary source of ATP ...
... not the mitochondria • For intense exercise of 1 to 2 minutes (e.g., jumping rope) duration, glycolysis provides the primary source of ATP ...
CHAPTER TWO ATOMS, MOLECULES, AND IONS
... The proton and neutron have similar mass with the mass of the neutron slightly larger than that of the proton. Each of these particles has a mass approximately 1800 times greater than that of an electron. The combination of the protons and the neutrons in the nucleus makes up the bulk of the mass of ...
... The proton and neutron have similar mass with the mass of the neutron slightly larger than that of the proton. Each of these particles has a mass approximately 1800 times greater than that of an electron. The combination of the protons and the neutrons in the nucleus makes up the bulk of the mass of ...
Lecture 22 – New HW assignment – Anaerobic metabolism (continued) – Other sugars
... Converted to F6P by two-step pathway 1. Hexokinase converts mannose to mannose-6phosphate 2. Phosphomannose isomerase converts the aldose to ketose F6P. (the mechanism is similar to phosphoglucose isomerase with an enediolate ...
... Converted to F6P by two-step pathway 1. Hexokinase converts mannose to mannose-6phosphate 2. Phosphomannose isomerase converts the aldose to ketose F6P. (the mechanism is similar to phosphoglucose isomerase with an enediolate ...
(a) (b)
... the cycloxygenase-2 (COX-2) enzyme. High substrate concentrations reduce the efficacy of inhibition by these drugs. These drugs are ...
... the cycloxygenase-2 (COX-2) enzyme. High substrate concentrations reduce the efficacy of inhibition by these drugs. These drugs are ...
Prolonged starvation
... Figure 2. Structures of important compounds having high-energy phosphate bonds. ATP formed by substrate-level phosphorylation ...
... Figure 2. Structures of important compounds having high-energy phosphate bonds. ATP formed by substrate-level phosphorylation ...
Reaction of glycolysis
... •Reaction is catalyzed by pyruvate kinase •The double bond shift to the oxygen on carbon 2 and a hydrogen shifts to carbon 3 • This reaction is irreversible (control point) ...
... •Reaction is catalyzed by pyruvate kinase •The double bond shift to the oxygen on carbon 2 and a hydrogen shifts to carbon 3 • This reaction is irreversible (control point) ...
Product Data Sheet
... enzymes (suitable for vegetarians) which are specific for the digestion of starches, proteins, fats, and cellulose, a dietary fiber. ...
... enzymes (suitable for vegetarians) which are specific for the digestion of starches, proteins, fats, and cellulose, a dietary fiber. ...
No Slide Title
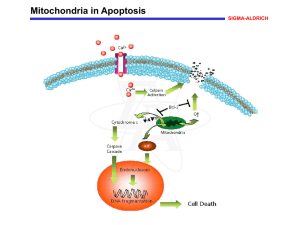
... Increases in cytosolic Ca2+ levels due to activation of ion channel-linked receptors, such as that for the excitatory amino acid neurotransmitter glutamic acid, can induce permeability transition (PT) of the mitochondrial membrane. PT constitutes the first rate-limiting event of the common pathway o ...
... Increases in cytosolic Ca2+ levels due to activation of ion channel-linked receptors, such as that for the excitatory amino acid neurotransmitter glutamic acid, can induce permeability transition (PT) of the mitochondrial membrane. PT constitutes the first rate-limiting event of the common pathway o ...
Derived copy of Bis2A 07.3 Oxidation of Pyruvate and the Citric Acid
... that reduce NAD+ to NADH and release carboxyl groups that form CO2 molecules. α-Ketoglutarate is the product of step three, and a succinyl group is the product of step four. CoA binds the succinyl group to form succinyl CoA. The enzyme that catalyzes step four is regulated by feedback inhibition of ...
... that reduce NAD+ to NADH and release carboxyl groups that form CO2 molecules. α-Ketoglutarate is the product of step three, and a succinyl group is the product of step four. CoA binds the succinyl group to form succinyl CoA. The enzyme that catalyzes step four is regulated by feedback inhibition of ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI
... Discuss any two hypotheses to explain the mechanism of formation of enzyme-substrate enzyme complex. ...
... Discuss any two hypotheses to explain the mechanism of formation of enzyme-substrate enzyme complex. ...
Lactanase - Vita Flex
... veterinarians at the nation's leading racetracks. It is now available to all horsemen for optimum support of horses involved in intensive exercise and performance. Anaerobic Metabolism is Used To Maintain Intensive Exercise When horses perform or exercise intensely, they quickly face a demand for en ...
... veterinarians at the nation's leading racetracks. It is now available to all horsemen for optimum support of horses involved in intensive exercise and performance. Anaerobic Metabolism is Used To Maintain Intensive Exercise When horses perform or exercise intensely, they quickly face a demand for en ...
Principles of BIOCHEMISTRY
... • Four kinases in glycolysis: steps 1,3,7, and 10, all of which require Mg2+ and have a similar mechanism. Prentice Hall c2002 ...
... • Four kinases in glycolysis: steps 1,3,7, and 10, all of which require Mg2+ and have a similar mechanism. Prentice Hall c2002 ...
Fermentation - cloudfront.net
... Where? In the mitochondria Steps? Kreb’s cycle and electron transport chain Glycolysis: -- In the cytoplasm -- Glucose is split into 2 pyruvate -- 2 ATP created ...
... Where? In the mitochondria Steps? Kreb’s cycle and electron transport chain Glycolysis: -- In the cytoplasm -- Glucose is split into 2 pyruvate -- 2 ATP created ...
Oxidative phosphorylation
Oxidative phosphorylation (or OXPHOS in short) is the metabolic pathway in which the mitochondria in cells use their structure, enzymes, and energy released by the oxidation of nutrients to reform ATP. Although the many forms of life on earth use a range of different nutrients, ATP is the molecule that supplies energy to metabolism. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation processes such as anaerobic glycolysis.During oxidative phosphorylation, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryotes, these redox reactions are carried out by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cells' intermembrane space. These linked sets of proteins are called electron transport chains. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called electron transport. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. This store of energy is tapped by allowing protons to flow back across the membrane and down this gradient, through a large enzyme called ATP synthase; this process is known as chemiosmosis. This enzyme uses this energy to generate ATP from adenosine diphosphate (ADP), in a phosphorylation reaction. This reaction is driven by the proton flow, which forces the rotation of a part of the enzyme; the ATP synthase is a rotary mechanical motor.Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging (senescence). The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.