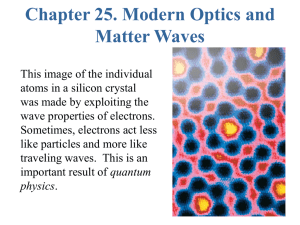
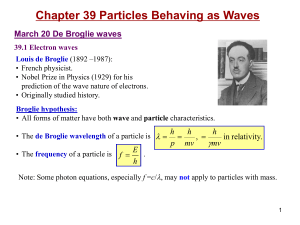
QUANTUM THEORY OF ATOMS AND MOLECULES
... etc.) represents the number of C atoms in the chain. The number of electrons is thus also Nc and by the Pauli principle the lowest Nc/2 levels will be doubly occupied in the ground state. How will the wavelength of the lowest energy electronic transition associated with the promotion of electrons ...
... etc.) represents the number of C atoms in the chain. The number of electrons is thus also Nc and by the Pauli principle the lowest Nc/2 levels will be doubly occupied in the ground state. How will the wavelength of the lowest energy electronic transition associated with the promotion of electrons ...
Chemistry for Changing Times 11th Edition Hill and Kolb
... absorbed by the electrons, they are promoted to a higher energy state (excited state). When an electron in an excited state returns to a lower energy state, it emits a photon of energy, which may be observed as light. ...
... absorbed by the electrons, they are promoted to a higher energy state (excited state). When an electron in an excited state returns to a lower energy state, it emits a photon of energy, which may be observed as light. ...
File
... valence shell) usually determine how an atom will react Atoms are stable when their outer energy level is full Atoms can gain or lose electrons to become stable ...
... valence shell) usually determine how an atom will react Atoms are stable when their outer energy level is full Atoms can gain or lose electrons to become stable ...
Chapt25_VGO
... Photo-electric effect (photons) Diffraction of electrons Classical physics was thought to describe the interaction of light with charges until there were discovered too many things that it could not explain. ...
... Photo-electric effect (photons) Diffraction of electrons Classical physics was thought to describe the interaction of light with charges until there were discovered too many things that it could not explain. ...
PHY4604–Introduction to Quantum Mechanics Fall 2004 Practice
... ii. deuterium (mass of nucleus 2× that of H). The mass which appears in the Bohr formula is the electron mass m, which is replaced by the reduced mass µ = mmp /(m+mp ) if one allows for the proton motion. Changing mp → 2mp doesn’t change much, so to a good approximation the photon wavelength doesn’t ...
... ii. deuterium (mass of nucleus 2× that of H). The mass which appears in the Bohr formula is the electron mass m, which is replaced by the reduced mass µ = mmp /(m+mp ) if one allows for the proton motion. Changing mp → 2mp doesn’t change much, so to a good approximation the photon wavelength doesn’t ...
Chemistry Exam Review
... sodium hydroxide reacts with hydrochloric acid to produce sodium chloride and water ...
... sodium hydroxide reacts with hydrochloric acid to produce sodium chloride and water ...
Electrons!
... The second quantum number, the angular momentum quantum number, l, can have integral values from 0 to (n-1) for each value of n. This quantum number defines the shape of the orbital. The value of l is generally designated by the letters, s, p, d and f, corresponding to l values of 0, 1, 2 and 3 ...
... The second quantum number, the angular momentum quantum number, l, can have integral values from 0 to (n-1) for each value of n. This quantum number defines the shape of the orbital. The value of l is generally designated by the letters, s, p, d and f, corresponding to l values of 0, 1, 2 and 3 ...
Semester 2 review questions
... 2. The state when all electrons of an atom are in the lowest possible energy levels. 3. When an electron jumps up to a higher energy level, the atom is in its ___. 4. The scientist who applied Einstein’s particle-wave theory to electrons. 5. The theory that it is impossible to know both the position ...
... 2. The state when all electrons of an atom are in the lowest possible energy levels. 3. When an electron jumps up to a higher energy level, the atom is in its ___. 4. The scientist who applied Einstein’s particle-wave theory to electrons. 5. The theory that it is impossible to know both the position ...
Pre-AP Chemistry
... 19. average atomic masses by abundance 20. Avogadro’s number and the mole 21. nuclear chemistry terms 22. types of nuclear reactions 23. radioactive decays 24. half-life rates 25. nuclear fission and fusion 26. nuclear waste and radiation 27. radiation properties 28. wave measurements 29. energy of ...
... 19. average atomic masses by abundance 20. Avogadro’s number and the mole 21. nuclear chemistry terms 22. types of nuclear reactions 23. radioactive decays 24. half-life rates 25. nuclear fission and fusion 26. nuclear waste and radiation 27. radiation properties 28. wave measurements 29. energy of ...
Sections 6.3-6.5
... Bohr Model of the Atom • Electrons move in certain, specific, circular orbitals • Smaller orbit = lower energy level • Assigned the allowable electron orbitals the principle quantum number, n. • 1st orbit= lowest energy: n=1 • 2nd orbit= 2nd lowest energy: n=2 ...
... Bohr Model of the Atom • Electrons move in certain, specific, circular orbitals • Smaller orbit = lower energy level • Assigned the allowable electron orbitals the principle quantum number, n. • 1st orbit= lowest energy: n=1 • 2nd orbit= 2nd lowest energy: n=2 ...
atoms
... occurring isotopes with masses of 78.0, 79.0, and 80.0 amu. What data is needed to calculate its average atomic mass? The natural abundance in percent of each isotope (Can you do these calculations?) ...
... occurring isotopes with masses of 78.0, 79.0, and 80.0 amu. What data is needed to calculate its average atomic mass? The natural abundance in percent of each isotope (Can you do these calculations?) ...
$doc.title
... Note for He2 (4 electrons), Pauli principle means two e’s in antibonding state as well as bonding state so no overall energy saving (inert gases – no bond - no He2) Mid-periodic table elements (half-filled orbitals) tend to have strongest bonds (e.g. melting points. etc.) ...
... Note for He2 (4 electrons), Pauli principle means two e’s in antibonding state as well as bonding state so no overall energy saving (inert gases – no bond - no He2) Mid-periodic table elements (half-filled orbitals) tend to have strongest bonds (e.g. melting points. etc.) ...
uncertainty, atom
... charge, and accelerating charges give off EM radiation (like an antenna), thus giving off energy. The electron would gradually lose all its energy. That doesn’t happen -- atoms are stable. ...
... charge, and accelerating charges give off EM radiation (like an antenna), thus giving off energy. The electron would gradually lose all its energy. That doesn’t happen -- atoms are stable. ...
Revision topic 1-3
... of the positively charged nucleus for the negatively charged electrons and repulsion between the electrons. The outer electrons (valence electrons) do not experience the full attraction of the positive nucleus because of the presence of inner electrons. They are shielded from the nucleus and repelle ...
... of the positively charged nucleus for the negatively charged electrons and repulsion between the electrons. The outer electrons (valence electrons) do not experience the full attraction of the positive nucleus because of the presence of inner electrons. They are shielded from the nucleus and repelle ...
topic 03 outline YT 2010 test
... Line spectrum: very specific wavelengths of light that atoms give off or gain o Each element has its own line spectrum, which can be used to identify that element. o The line spectrum must be related to energy transitions in the atom o ...
... Line spectrum: very specific wavelengths of light that atoms give off or gain o Each element has its own line spectrum, which can be used to identify that element. o The line spectrum must be related to energy transitions in the atom o ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.