* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Sections 6.3-6.5
Franck–Condon principle wikipedia , lookup
Molecular orbital wikipedia , lookup
Matter wave wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Electron scattering wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Particle in a box wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Hydrogen atom wikipedia , lookup
Tight binding wikipedia , lookup
Atomic orbital wikipedia , lookup
Atomic theory wikipedia , lookup
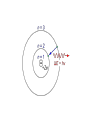
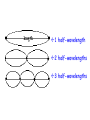
Sections 6.3-6.5 The Bohr Model, Wave Model, and Quantum Model Objectives • • • • • • Draw Bohr Models Describe the wave behavior of matter Arrange electrons in an atom Identify energy levels Apply Heisenberg’s Uncertainty Principle Describe the quantum mechanical model Key Terms • • • • • Ground state Excited states Matter waves momentum Uncertainty principle • • • • • • Wave functions Probability density Electron density Orbitals Electron shell subshell Bohr Model of the Atom • In 1913 Niels Bohr proposed quantum model for the H atom • Bohr proposed H atom has only certain allowable energy states • Lowest state= ground state • Gaining energy = excited state Bohr Model of the Atom • Electrons move in certain, specific, circular orbitals • Smaller orbit = lower energy level • Assigned the allowable electron orbitals the principle quantum number, n. • 1st orbit= lowest energy: n=1 • 2nd orbit= 2nd lowest energy: n=2 Bohr Model of the Atom • Energy is added to an atomelectron moves to higher energy level • Electron in “excited state” drops to a lower energy orbit emits a photon E = E higher-energy orbit – E lower-energy orbit= E photon= h Bohr Model of the Atom • Problems with Bohr’s model – Only explained H – Did not explain why electrons should only be allowed certain, specific energy levels De Broglie • 1924 • Electrons, like light also had a particle-wave dual nature • Only multiples of half wavelengths are allowed in circular orbits 1 half-wavelength 2 half-wavelengths 3 half-wavelengths De Broglie • Formulated an equation for the wavelength, mass, and velocity of a particle h mv Heisenberg Uncertainty Principle – Fundamentally impossible to know precisely both the velocity AND position of a particle at the same time. – Cannot measure an object without disturbing it Quantum Mechanical Model • 1926 • Schrödinger • Limited electrons to only certain energy levels – Atomic orbital: 3 dimensional area around the nucleus that predicts the 90 % PROBABLE location of an electron Electron Density Diagram Quantum Mechanical Model • Assigns principal quantum numbers (n) relative to sizes and energies of orbitals • (n) specifies atom’s major energy levels= principle energy levels • Lowest level= ground state= n= 1 • H has 7 energy levels, n= 1 to 7 Quantum Mechanical Model • Principal energy levels contain energy sublevels • Principal energy level 1 has 1 sublevel • Principal energy level 2 has 2 sublevels • Principal energy level 3 has 3 sublevels Energy Sublevels • s, p, d, and f • Labeled according to shapes of orbitals • s = spherical • p = dumbbell • d and f = not all have same shape s and p orbitals Three p orbitals d orbitals Energy Sublevels • Each orbital contains 2 electron at most • Principal energy level 1 has 1 sublevel: 1s orbital • Principal energy level 2 has 2 sublevels: 2s and 2p • 2p sublevel has 3 dumbbell-shaped p orbitals (2px, 2py, and 2pz) • Principal energy level 3 has 3 sublevels: 3s, 3p, and 3d • d sublevels have 5 orbitals • Principal energy level 4 has 4 sublevels: 4s, 4p, 4d, and 4f • f sublevels have 7 orbitals