L3 - eLearning
... Recap – Last Lecture • Bohr model of the atom: electrons occupy orbits of certain energies. • Evidence of this from atomic spectra in which wavelength of light is related to energy difference between orbits. ...
... Recap – Last Lecture • Bohr model of the atom: electrons occupy orbits of certain energies. • Evidence of this from atomic spectra in which wavelength of light is related to energy difference between orbits. ...
Document
... • An electron is characterized by quantum numbers. These can be measured without uncertainty. • The quantum number n labels the energy level En . • The lowest energy level with n = 1 is sharp (E= 0), because an atom is stable. One can take an infinite time (t = ) to determine its energy and there ...
... • An electron is characterized by quantum numbers. These can be measured without uncertainty. • The quantum number n labels the energy level En . • The lowest energy level with n = 1 is sharp (E= 0), because an atom is stable. One can take an infinite time (t = ) to determine its energy and there ...
• Quantum physics explains the energy levels of atoms with
... • An electron is characterized by quantum numbers. These can be measured without uncertainty. • The quantum number n labels the energy level En . • The lowest energy level with n = 1 is sharp (E= 0), because an atom is stable. One can take an infinite time (t = ) to determine its energy and there ...
... • An electron is characterized by quantum numbers. These can be measured without uncertainty. • The quantum number n labels the energy level En . • The lowest energy level with n = 1 is sharp (E= 0), because an atom is stable. One can take an infinite time (t = ) to determine its energy and there ...
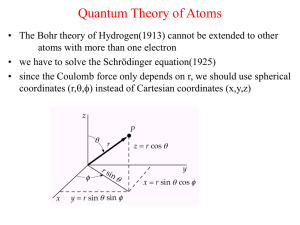
Quantum Theory of Atoms
... distance r of an electron from the nucleus • l is the orbital quantum number and the angular momentum of the electron is given by L=[l(l+1)]1/2 ħ • m is the magnetic quantum number and the component of the angular momentum along the z-axis is Lz = m ħ ...
... distance r of an electron from the nucleus • l is the orbital quantum number and the angular momentum of the electron is given by L=[l(l+1)]1/2 ħ • m is the magnetic quantum number and the component of the angular momentum along the z-axis is Lz = m ħ ...
5 - BrainMass
... 5.72) Using the values from Thermodynamics Quantities for Selected Substances at 298.15 K (25°C), calculate the value of ΔH° for each of the following reactions: a. N2O4 (g) + 4 H2 (g) N2 (g) + 4 H2O (g) b. 2 KOH(s) + CO2 (g) K2CO3(s) + H2O (g) c. SO2 (g) + 2 H2S (g) 3/8 S8(s) + 2 H2O (g) d. F ...
... 5.72) Using the values from Thermodynamics Quantities for Selected Substances at 298.15 K (25°C), calculate the value of ΔH° for each of the following reactions: a. N2O4 (g) + 4 H2 (g) N2 (g) + 4 H2O (g) b. 2 KOH(s) + CO2 (g) K2CO3(s) + H2O (g) c. SO2 (g) + 2 H2S (g) 3/8 S8(s) + 2 H2O (g) d. F ...
Electronic Structure of Atoms
... • Two spots were found: one with the electrons spinning in one direction and one with the electrons spinning in the opposite direction. Since electron spin (electron as a tiny sphere spinning on its own axis) is quantized, • we define ms = spin magnetic quantum number = ± ½. Pauli’s exclusion princi ...
... • Two spots were found: one with the electrons spinning in one direction and one with the electrons spinning in the opposite direction. Since electron spin (electron as a tiny sphere spinning on its own axis) is quantized, • we define ms = spin magnetic quantum number = ± ½. Pauli’s exclusion princi ...
sch4u-quantumtheory
... A wave function with a given set of these three quantum numbers is called an atomic orbital In quantum mechanics the atomic orbitals require three integer quantum numbers to completely describe the energy and the shape of the 3-D space occupied by the electron (n, l, and ml) ...
... A wave function with a given set of these three quantum numbers is called an atomic orbital In quantum mechanics the atomic orbitals require three integer quantum numbers to completely describe the energy and the shape of the 3-D space occupied by the electron (n, l, and ml) ...
Energy Levels and Light Absorption
... • Consider an electron in an atom with quantum number n = n1. How many electrons in this atom can have the quantum number n1? n1 • Pauli exclusion says no two electrons can be in exactly the same state ...
... • Consider an electron in an atom with quantum number n = n1. How many electrons in this atom can have the quantum number n1? n1 • Pauli exclusion says no two electrons can be in exactly the same state ...
Electronic Structure of Atoms
... the same set of 4 quantum numbers. Therefore, two electrons in the same orbital must have opposite spins. ...
... the same set of 4 quantum numbers. Therefore, two electrons in the same orbital must have opposite spins. ...
CHAPTER 10 - NUCLEAR PHYSICS
... When chemical bonds are formed, electrons are shared between atoms or they are transferred from one atom to another to create a positive and negative ion. When chemical bonds are formed due to electron transfer, this process is called ionic bonding. Chemical bonds are formed spontaneously when elect ...
... When chemical bonds are formed, electrons are shared between atoms or they are transferred from one atom to another to create a positive and negative ion. When chemical bonds are formed due to electron transfer, this process is called ionic bonding. Chemical bonds are formed spontaneously when elect ...
NUCLEAR PHYSICS
... An energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one orbital to another by emission or absorption of a quantum of energy, in the form of a photon. Knowledge of the electron configuration of different atoms is useful in understandi ...
... An energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one orbital to another by emission or absorption of a quantum of energy, in the form of a photon. Knowledge of the electron configuration of different atoms is useful in understandi ...
Details
... Structure of the atom, the early atomic theories, theories of electromagnetic radiation, Plank theory, Bohr theory. Quantum theory, Schrodinger wave equation, quantum numbers, shapes of orbitals and electronic configuration. Periodic classification of the elements and periodic relationship among the ...
... Structure of the atom, the early atomic theories, theories of electromagnetic radiation, Plank theory, Bohr theory. Quantum theory, Schrodinger wave equation, quantum numbers, shapes of orbitals and electronic configuration. Periodic classification of the elements and periodic relationship among the ...
Advanced Chemistry - Forestville Middle
... The arrangement of electrons within the orbitals of an atom is known as the electron configuration. The most stable arrangement is called the ground-state electron configuration. This is the configuration where all of the electrons in an atom reside in the lowest energy orbitals possible. Bearing in ...
... The arrangement of electrons within the orbitals of an atom is known as the electron configuration. The most stable arrangement is called the ground-state electron configuration. This is the configuration where all of the electrons in an atom reside in the lowest energy orbitals possible. Bearing in ...
Review II
... A. Write the correct skeletal structure for the molecule B. Calculate the total number of valence electrons C. Distribute electrons among atoms giving each an octet (duet) D. If any atoms the lack an octet, form double or triple bonds as necessary E. Exception to the octet rule 1. Duet rule for hydr ...
... A. Write the correct skeletal structure for the molecule B. Calculate the total number of valence electrons C. Distribute electrons among atoms giving each an octet (duet) D. If any atoms the lack an octet, form double or triple bonds as necessary E. Exception to the octet rule 1. Duet rule for hydr ...
III. Quantum Model of the Atom
... C. Quantum Numbers Pauli Exclusion Principle No two electrons in an atom can have the same 4 quantum numbers. Each e- has a unique “address”: 1. Principal # 2. Ang. Mom. # 3. Magnetic # 4. Spin # ...
... C. Quantum Numbers Pauli Exclusion Principle No two electrons in an atom can have the same 4 quantum numbers. Each e- has a unique “address”: 1. Principal # 2. Ang. Mom. # 3. Magnetic # 4. Spin # ...
Chapter 6. Electronic Structure of Atoms
... • values of l begin at 0 and increase to n – 1 • use letters for l (s, p, d, and f for l = 0, 1, 2, and 3), refer to the s, p, d, and f orbitals • defines the shape of the orbital • Magnetic quantum number, ml • depends on l • has integer values between –l and +l • are (2l+1) possible values of ml • ...
... • values of l begin at 0 and increase to n – 1 • use letters for l (s, p, d, and f for l = 0, 1, 2, and 3), refer to the s, p, d, and f orbitals • defines the shape of the orbital • Magnetic quantum number, ml • depends on l • has integer values between –l and +l • are (2l+1) possible values of ml • ...
1. Larger a
... reasons for the additional complication are: (1) All other atoms, being more massive than hydrogen, contain more than a single electron, and those electrons interact with one another, in addition to interacting with the positively charged atomic nucleus; (2) It turns out that further quantum numbers ...
... reasons for the additional complication are: (1) All other atoms, being more massive than hydrogen, contain more than a single electron, and those electrons interact with one another, in addition to interacting with the positively charged atomic nucleus; (2) It turns out that further quantum numbers ...
Solution - UMD Physics
... a. What are the possible values obtained in a measurement of A? (2) b. Does a state exist in which both the results of a measurement of energy E and observable A can be predicted with certainty? Why or why not? (2) c. Two measurements of A are carried out, separated in time by t. If the result of th ...
... a. What are the possible values obtained in a measurement of A? (2) b. Does a state exist in which both the results of a measurement of energy E and observable A can be predicted with certainty? Why or why not? (2) c. Two measurements of A are carried out, separated in time by t. If the result of th ...
Chem312 Au03 Problem Set 4
... This paper reports unusual two-coordinate nickel complexes. The nickel can only bind two ligands because the ligands are so huge (also called bulky or sterically large or sterically encumbered). Figures 1 and 3 show the large size of the ligands. They are the results of Xray diffraction experiments ...
... This paper reports unusual two-coordinate nickel complexes. The nickel can only bind two ligands because the ligands are so huge (also called bulky or sterically large or sterically encumbered). Figures 1 and 3 show the large size of the ligands. They are the results of Xray diffraction experiments ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.