Chapter 5 Electrons in Atoms
... Electron Configurations… …are the way electrons are arranged in various orbitals around the nuclei of atoms. Three rules tell us how: 1) Aufbau principle - electrons enter the lowest energy first. • This causes difficulties because of the overlap of orbitals of different energies – follow the diagr ...
... Electron Configurations… …are the way electrons are arranged in various orbitals around the nuclei of atoms. Three rules tell us how: 1) Aufbau principle - electrons enter the lowest energy first. • This causes difficulties because of the overlap of orbitals of different energies – follow the diagr ...
For a “black body” - The University of Sheffield
... “UV catastrophe” •First experimental “anomaly” to be explained by the need for a quantum theory (1900) •“h” originally introduced by Planck purely as an empirical constant to fit data………………………… ...
... “UV catastrophe” •First experimental “anomaly” to be explained by the need for a quantum theory (1900) •“h” originally introduced by Planck purely as an empirical constant to fit data………………………… ...
orbital quantum number
... Schrödinger's equation… …but a positive energy means the electron is not bound, so we don't have a electron in the hydrogen atom. The only possible negative (bound electron) energies are those given by the equation above. None of this information is new; we have seen it all before. ...
... Schrödinger's equation… …but a positive energy means the electron is not bound, so we don't have a electron in the hydrogen atom. The only possible negative (bound electron) energies are those given by the equation above. None of this information is new; we have seen it all before. ...
Quantum-Electrodynamics and the Magnetic Moment of the
... The new Hamiltonian is superior to the original one in essentially three ways: it involves the experimental electron mass, rather than the unobservable mechanical mass; an electron now interacts with the radiation field only in the presence of an external field, that is, only an accelerated electron ...
... The new Hamiltonian is superior to the original one in essentially three ways: it involves the experimental electron mass, rather than the unobservable mechanical mass; an electron now interacts with the radiation field only in the presence of an external field, that is, only an accelerated electron ...
1) - Kurt Niedenzu
... 32) The increase in atomic radius of each successive element within a group is primarily due to an increase in the number of a) neutrons in the nucleus b) electrons in the outermost shell c) unpaired electrons d) occupied principal energy levels 33) Elements that have properties of both metals and n ...
... 32) The increase in atomic radius of each successive element within a group is primarily due to an increase in the number of a) neutrons in the nucleus b) electrons in the outermost shell c) unpaired electrons d) occupied principal energy levels 33) Elements that have properties of both metals and n ...
Quantum mechanics
... it is a whole particle that is being detected. It is particle-like. • The matter wave commands the particle where to go and arrive in different positions with the associated probability. ...
... it is a whole particle that is being detected. It is particle-like. • The matter wave commands the particle where to go and arrive in different positions with the associated probability. ...
Unit 3 Test - hrsbstaff.ednet.ns.ca
... B. True and False (1 point each) ___ Combustibility is the ability of a substance to react with acids ___ Sugar disappearing in water is an example of a solution ___ Raisins in Raisin Bran are an example of a solution ___ Lighting a test tube of acetylene gas is an example of a reaction with acid __ ...
... B. True and False (1 point each) ___ Combustibility is the ability of a substance to react with acids ___ Sugar disappearing in water is an example of a solution ___ Raisins in Raisin Bran are an example of a solution ___ Lighting a test tube of acetylene gas is an example of a reaction with acid __ ...
Electronic Structure
... it emits a quantum of energy E in radiation of definite frequency, . Since E for the change from E2 to E1 is always the same in a given atom, and h is a constant , must be a constant. Therefore, radiation always has the same energy and is always of the same frequency for this particular electro ...
... it emits a quantum of energy E in radiation of definite frequency, . Since E for the change from E2 to E1 is always the same in a given atom, and h is a constant , must be a constant. Therefore, radiation always has the same energy and is always of the same frequency for this particular electro ...
CHEM-UA 127: Advanced General Chemistry
... Let us now consider two identical particles, one of which has coordinate and spin x1 and the other has coordinate and spin x2 . The question now arises as to which particle do we assign to x1 and which do we assign to x2 ? In fact, if the particles are indistinguishable, then is does not make sense ...
... Let us now consider two identical particles, one of which has coordinate and spin x1 and the other has coordinate and spin x2 . The question now arises as to which particle do we assign to x1 and which do we assign to x2 ? In fact, if the particles are indistinguishable, then is does not make sense ...
Article3-Dirac - Inframatter Research Center
... Another small effect is the variation in charge distribution across the face of the nucleus. Points where the charge is more concentrated would lead to higher velocities in the orbiting electron, while dilute regions would produce a slower velocity. This effect averages out, except the electron may ...
... Another small effect is the variation in charge distribution across the face of the nucleus. Points where the charge is more concentrated would lead to higher velocities in the orbiting electron, while dilute regions would produce a slower velocity. This effect averages out, except the electron may ...
Introduction to Spectroscopy
... • H atom – simplest: En = -13.6/n2 eV; transitions between levels; absorption/emission lines • Classify E levels into 4 types: – Electronic – due to orbital motion of e-; lowest = ground state – quantum number n, with typical DE ~ eV (remember kBT ~ 1/40 eV at Room T); transitions produces uv-vis sp ...
... • H atom – simplest: En = -13.6/n2 eV; transitions between levels; absorption/emission lines • Classify E levels into 4 types: – Electronic – due to orbital motion of e-; lowest = ground state – quantum number n, with typical DE ~ eV (remember kBT ~ 1/40 eV at Room T); transitions produces uv-vis sp ...
Chapter 7: Quantum Mechanical Model of Atom
... • Max Planck proposed that matter & energy are not distinct in nature; they can be looked upon as one in the same. • Energy is only emitted in discrete packets called quanta. ...
... • Max Planck proposed that matter & energy are not distinct in nature; they can be looked upon as one in the same. • Energy is only emitted in discrete packets called quanta. ...
biol 1406 chapter 3: water
... Determine if the statement is true. If it is not, rewrite the italicized part to make it true. 1. An element is a substance that can be broken down into simpler substances. ______________________ 2. On Earth, 90 elements occur naturally. ________________________________________ 3. Only four elements ...
... Determine if the statement is true. If it is not, rewrite the italicized part to make it true. 1. An element is a substance that can be broken down into simpler substances. ______________________ 2. On Earth, 90 elements occur naturally. ________________________________________ 3. Only four elements ...
The Chemical Context of Life
... of its location or structure, there are many kinds…not just gravitational PE! • The electrons of an atom differ in their amounts of potential energy • An electron’s state of potential energy is called its energy level, or electron shell* * “Shell” is fraught with misconception—but biologists often u ...
... of its location or structure, there are many kinds…not just gravitational PE! • The electrons of an atom differ in their amounts of potential energy • An electron’s state of potential energy is called its energy level, or electron shell* * “Shell” is fraught with misconception—but biologists often u ...
Chapter 2 Name___________________________________
... A) Covalent bonds involve the sharing of protons between atoms, and ionic bonds involve the sharing of neutrons between atoms. B) Covalent bonds involve the sharing of protons between atoms, and ionic bonds involve the sharing of electrons between atoms. C) Covalent bonds involve the sharing of neut ...
... A) Covalent bonds involve the sharing of protons between atoms, and ionic bonds involve the sharing of neutrons between atoms. B) Covalent bonds involve the sharing of protons between atoms, and ionic bonds involve the sharing of electrons between atoms. C) Covalent bonds involve the sharing of neut ...
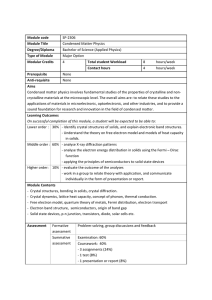
Module code SP-2306 Module Title Condensed Matter Physics
... Condensed matter physics involves fundamental studies of the properties of crystalline and noncrystalline materials at the microscopic level. The overall aims are: to relate these studies to the applications of materials in microelectronic, optoelectronic, and other industries, and to provide a so ...
... Condensed matter physics involves fundamental studies of the properties of crystalline and noncrystalline materials at the microscopic level. The overall aims are: to relate these studies to the applications of materials in microelectronic, optoelectronic, and other industries, and to provide a so ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.