SCH4U - Unit 1
... Schrodinger (1924) postulated that sometimes electrons behave as particles, and sometimes like waves. Because of this we cannot measure both the position and velocity of an electron at the same time. This exclusion is referred to as the Pauli Exclusion Principle. What this really means is that we ca ...
... Schrodinger (1924) postulated that sometimes electrons behave as particles, and sometimes like waves. Because of this we cannot measure both the position and velocity of an electron at the same time. This exclusion is referred to as the Pauli Exclusion Principle. What this really means is that we ca ...
Atomic
... atomic theory that he created using the laws of matter and previously known atomic theory 1. All matter is composed of atoms 2. All atoms of a given element are identical in size, mass, and other properties 3. Atoms can not be divided, created or destroyed 4. Atoms of different elements combine in s ...
... atomic theory that he created using the laws of matter and previously known atomic theory 1. All matter is composed of atoms 2. All atoms of a given element are identical in size, mass, and other properties 3. Atoms can not be divided, created or destroyed 4. Atoms of different elements combine in s ...
Chemistry 199 - Oregon State chemistry
... What is a Lewis base? What is a Lewis acid? Let me start by stating that we are familiar with many bases and acids. Those we know to be bases are Lewis bases and those we know to be acids are Lewis acids. Our previous ideas of bases and acids came from Arrhenius, Bronsted, and Lowry. These ideas inv ...
... What is a Lewis base? What is a Lewis acid? Let me start by stating that we are familiar with many bases and acids. Those we know to be bases are Lewis bases and those we know to be acids are Lewis acids. Our previous ideas of bases and acids came from Arrhenius, Bronsted, and Lowry. These ideas inv ...
IB Definitions
... An exothermic reaction is one in which there is an overall negative enthalpy change (heat is evolved) An endothermic reaction is one in which there is an overall postive enthalpy change (heat is absorbed) The standard enthalpy change of a reaction is the enthalpy change when one mole of reactants is ...
... An exothermic reaction is one in which there is an overall negative enthalpy change (heat is evolved) An endothermic reaction is one in which there is an overall postive enthalpy change (heat is absorbed) The standard enthalpy change of a reaction is the enthalpy change when one mole of reactants is ...
Chem. 1A Week 11 Discussion Notes Dr. Mack/S12 Page 1 of 5 B
... None of the atoms in structures 5 or 6 has a formal charge, so these two structures are predicted to be stable. In this case, structures 5 and 6 are said to be “isomers,” both with the molecular formula C2H6O. (Structure 5 is ethyl alcohol and structure 6 is dimethyl ether.) None of the atoms in ...
... None of the atoms in structures 5 or 6 has a formal charge, so these two structures are predicted to be stable. In this case, structures 5 and 6 are said to be “isomers,” both with the molecular formula C2H6O. (Structure 5 is ethyl alcohol and structure 6 is dimethyl ether.) None of the atoms in ...
phys3313-fall12
... In the limits where classical and quantum theories should agree, the quantum theory must produce the classical results. PHYS 3313-001, Fall 2012 Dr. Jaehoon Yu ...
... In the limits where classical and quantum theories should agree, the quantum theory must produce the classical results. PHYS 3313-001, Fall 2012 Dr. Jaehoon Yu ...
protons
... Chemical combination of 2 or more elements Water = H2O A compound can behave differently than the elements that make it up ...
... Chemical combination of 2 or more elements Water = H2O A compound can behave differently than the elements that make it up ...
Balmer Series
... Universe is made of hydrogen. Emission or absorption processes in hydrogen give rise to series, which are sequences of lines corresponding to atomic transitions, each ending or beginning with the same atomic state in hydrogen. Thus, for example, the Balmer Series involves transitions starting (for a ...
... Universe is made of hydrogen. Emission or absorption processes in hydrogen give rise to series, which are sequences of lines corresponding to atomic transitions, each ending or beginning with the same atomic state in hydrogen. Thus, for example, the Balmer Series involves transitions starting (for a ...
File
... 2) Which of the following statements is TRUE? A) A scientific law is fact. B) Once a theory is constructed, it is considered fact. C) A hypothesis is speculation that is difficult to test. D) An observation explains why nature does something. E) A scientific law summarizes a series of related observ ...
... 2) Which of the following statements is TRUE? A) A scientific law is fact. B) Once a theory is constructed, it is considered fact. C) A hypothesis is speculation that is difficult to test. D) An observation explains why nature does something. E) A scientific law summarizes a series of related observ ...
Lecture 5: The Hydrogen Atom (continued). In the previous lecture
... With high resolution spectrometers one finds many spectral lines split into two. This is called fine structure. The first attempt at an explanation of the fine structure was made by Sommerfeld in 1916, even before the birth of quantum mechanics. Developing the Bohr theory of the hydrogen atom to a r ...
... With high resolution spectrometers one finds many spectral lines split into two. This is called fine structure. The first attempt at an explanation of the fine structure was made by Sommerfeld in 1916, even before the birth of quantum mechanics. Developing the Bohr theory of the hydrogen atom to a r ...
+ 2 HCL(aq) CaCl2(aq) + H2O(l) + CO2(g)
... Chemical Formula: States what elements a compound contains and the exact number of atoms of these elements. Oxidation Number: positive or negative number on the periodic table that indicates how many electrons an element has gained, lost or shared when bonding with another element. Polyatomic Atom: ...
... Chemical Formula: States what elements a compound contains and the exact number of atoms of these elements. Oxidation Number: positive or negative number on the periodic table that indicates how many electrons an element has gained, lost or shared when bonding with another element. Polyatomic Atom: ...
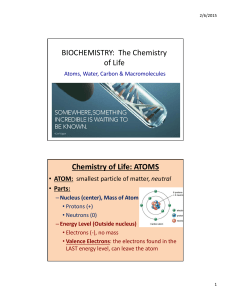
C10J ATOMIC STRUCTURE (6 lectures)
... You are encouraged at this early stage in the degree programme, not to form, or create, an imaginary boundary which separates the different disciplines of Chemistry, but to search for and make the links which will make understanding the different topics more enjoyable. Atomic structure refers to the ...
... You are encouraged at this early stage in the degree programme, not to form, or create, an imaginary boundary which separates the different disciplines of Chemistry, but to search for and make the links which will make understanding the different topics more enjoyable. Atomic structure refers to the ...
Lecture 13: Heisenberg and Uncertainty
... The more accurately we know the energy of a body, the less accurately we know how long it possessed that energy The energy can be known with perfect precision (DE = 0), only if the measurement is made over an infinite period of time (Dt = ∞) ...
... The more accurately we know the energy of a body, the less accurately we know how long it possessed that energy The energy can be known with perfect precision (DE = 0), only if the measurement is made over an infinite period of time (Dt = ∞) ...
Quantum Mechanics-Atomic, molecular, and optical physics
... Classical Monte-Carlo methods for the dynamics of electrons can be described as semi-classical in that the initial conditions are calculated using a fully quantum treatment, but all further treatment is classical.[2]:871 Isolated atoms and molecules Atomic, Molecular and Optical physics frequently c ...
... Classical Monte-Carlo methods for the dynamics of electrons can be described as semi-classical in that the initial conditions are calculated using a fully quantum treatment, but all further treatment is classical.[2]:871 Isolated atoms and molecules Atomic, Molecular and Optical physics frequently c ...
Lecture. Photoelectric Effect
... “Although surely the correct description of the electromagnetic field is a quantum one, just as surely the vast majority of optical phenomena are equally well described by a semiclassical theory, with atoms quantized but with a classical field. ... The first experimental example of a manifestly quan ...
... “Although surely the correct description of the electromagnetic field is a quantum one, just as surely the vast majority of optical phenomena are equally well described by a semiclassical theory, with atoms quantized but with a classical field. ... The first experimental example of a manifestly quan ...
Chapter 3. The Structure of the Atom
... could see through the challenges classical physics faced and came up with imaginative solutions based on the nascent quantum theory. When considering the failure of the atomic classical model Bohr proposed four general assumptions or postulates, which he could then use to explain much of the experim ...
... could see through the challenges classical physics faced and came up with imaginative solutions based on the nascent quantum theory. When considering the failure of the atomic classical model Bohr proposed four general assumptions or postulates, which he could then use to explain much of the experim ...
Chapter 2 (Hill/Petrucci/McCreary/Perry This chapter deals with
... 2. in chemical reactions, atoms are neither created nor destroyed 3. atoms of each element have unique properties - all atoms of a given atom are identical and have identical masses and other properties 4. chemical reactions involve the uniting or the separation of atoms of different elements Dalton ...
... 2. in chemical reactions, atoms are neither created nor destroyed 3. atoms of each element have unique properties - all atoms of a given atom are identical and have identical masses and other properties 4. chemical reactions involve the uniting or the separation of atoms of different elements Dalton ...
summer learning G10
... 12. Combine each pair of ions to get the formula of the compound they form and give the name of the compound formed https://youtu.be/C6cTM8jRY7o?t=114 ...
... 12. Combine each pair of ions to get the formula of the compound they form and give the name of the compound formed https://youtu.be/C6cTM8jRY7o?t=114 ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.