Development of Bohr model due to atomic emission spectra of some
... revolve around the nucleus on distinct radii, each of those radii due to their distance from the nucleus represent an energy level. These energy levels were not continuously, but existed only at discrete values. Each of these energy packs was called a quantum. The idea of quantized radiation was fir ...
... revolve around the nucleus on distinct radii, each of those radii due to their distance from the nucleus represent an energy level. These energy levels were not continuously, but existed only at discrete values. Each of these energy packs was called a quantum. The idea of quantized radiation was fir ...
Document
... http://images.encarta.msn.com/xrefmedia/aencm ed/targets/illus/ilt/T046738A.gif 12. Molecules and Compounds Molecule – two or more atoms held together by chemical bonds Compound – two or more different kinds of atoms chemically bonded together 12. Chemical Bonds Electron shells, or energy levels, su ...
... http://images.encarta.msn.com/xrefmedia/aencm ed/targets/illus/ilt/T046738A.gif 12. Molecules and Compounds Molecule – two or more atoms held together by chemical bonds Compound – two or more different kinds of atoms chemically bonded together 12. Chemical Bonds Electron shells, or energy levels, su ...
CHEM 532 Physical Chemistry II (Quantum Chemistry) Fall 2013
... the variation method, time independent perturbation theory, degenerate perturbation theory, the anharmonic oscillator VIII. The Helium atom electron spin, ground state of He, excited electronic states of He, spin eigenfunctions of He IX. Many-electron wavefunctions indistinguishable particles, the P ...
... the variation method, time independent perturbation theory, degenerate perturbation theory, the anharmonic oscillator VIII. The Helium atom electron spin, ground state of He, excited electronic states of He, spin eigenfunctions of He IX. Many-electron wavefunctions indistinguishable particles, the P ...
2 - Physics at Oregon State University
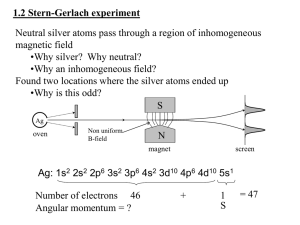
... •How can a neutral atom interact with a magnetic field? •Let’s derive it classically from intro-course principles •What does a simple magnetic dipole look like? •What does the energy look like? •What will the force be and why does the B need to be inhomogeneous? •How do we relate this to angular mom ...
... •How can a neutral atom interact with a magnetic field? •Let’s derive it classically from intro-course principles •What does a simple magnetic dipole look like? •What does the energy look like? •What will the force be and why does the B need to be inhomogeneous? •How do we relate this to angular mom ...
Chapter 2 - Molecules of Life (Biochemistry) Periodic Table of
... has same # of protons and electrons so charge = 0" Atomic number = # protons! Mass number = "# protons + # neutrons! ...
... has same # of protons and electrons so charge = 0" Atomic number = # protons! Mass number = "# protons + # neutrons! ...
name
... NAME _______________________________________ PERIOD _______________ DATE ___________ CHAPTER 5 CHARACTERISTICS OF ELEMENTS Use a periodic table of the elements to help you answer the following questions. 1. a) ...
... NAME _______________________________________ PERIOD _______________ DATE ___________ CHAPTER 5 CHARACTERISTICS OF ELEMENTS Use a periodic table of the elements to help you answer the following questions. 1. a) ...
PHYS-201 LAB-03 Bohr`s Model and Emission Spectra of Hydrogen
... It should be emphasized that eq.[6] models a singly ionized helium atom and not a neutral helium atom. Therefore, every line that you observe in the helium spectrum can not be described by eq. [7]. According to [6], the energy required to remove the second electron from the He+ is 54.4eV as experime ...
... It should be emphasized that eq.[6] models a singly ionized helium atom and not a neutral helium atom. Therefore, every line that you observe in the helium spectrum can not be described by eq. [7]. According to [6], the energy required to remove the second electron from the He+ is 54.4eV as experime ...
Abstract
... that it is not continuous but atomistic, not absolute but relative, not classical but quantized. In the ensuing century his heuristic hypotheses were con rmed as facts. They de ne what might be called the \atomic world view." Today we stand on the threshold of a new era: the information age. Far fro ...
... that it is not continuous but atomistic, not absolute but relative, not classical but quantized. In the ensuing century his heuristic hypotheses were con rmed as facts. They de ne what might be called the \atomic world view." Today we stand on the threshold of a new era: the information age. Far fro ...
hydrogen
... where the principal quantum number is n = 1, 2, 3, … and n > l. The negative sign indicates that the electron is bound to the nucleus. If the energy were to become positive, then the electron would no longer be a bound particle and the total energy would no longer be quantized. The quantized energy ...
... where the principal quantum number is n = 1, 2, 3, … and n > l. The negative sign indicates that the electron is bound to the nucleus. If the energy were to become positive, then the electron would no longer be a bound particle and the total energy would no longer be quantized. The quantized energy ...
Grade 11 Chemistry E.. - hrsbstaff.ednet.ns.ca
... (a) group 6A, period 4 (b) the alkaline earth metals group, period 5 (c) the halogen group, period 5 (d) the oxygen group, period 3 ...
... (a) group 6A, period 4 (b) the alkaline earth metals group, period 5 (c) the halogen group, period 5 (d) the oxygen group, period 3 ...
MOLECULAR ORBITAL THEORY AND BONDING NOTES
... In an attempt to handle the problem of calculating a molecular wavefunction, we must break it down somewhat. The most popular approach is to assume that the wavefunction for all the electrons in a molecule can be written as a product of N one-electron wavefunctions. The square of the total wavefunct ...
... In an attempt to handle the problem of calculating a molecular wavefunction, we must break it down somewhat. The most popular approach is to assume that the wavefunction for all the electrons in a molecule can be written as a product of N one-electron wavefunctions. The square of the total wavefunct ...
Electrons
... • When electricity is passed through a gas, the atoms will emit lines of different frequencies, which serves as its “fingerprint” ...
... • When electricity is passed through a gas, the atoms will emit lines of different frequencies, which serves as its “fingerprint” ...
Chapter 2
... • Atoms of the various elements differ in number of subatomic particles • An element’s atomic number is the number of protons in its nucleus • An element’s mass number is the sum of protons plus neutrons in the nucleus • Atomic mass, the atom’s total mass, can be approximated by the mass number Copy ...
... • Atoms of the various elements differ in number of subatomic particles • An element’s atomic number is the number of protons in its nucleus • An element’s mass number is the sum of protons plus neutrons in the nucleus • Atomic mass, the atom’s total mass, can be approximated by the mass number Copy ...
UVM Physics MS: Comprehensive Exam Date: Saturday January 11, 2013 Time:
... decays at infinity.) Write down the normalization condition. (b) Impose the correct boundary conditions at the origin. Show that in addition to the wavefunction continuity equation ψ(+0) = ψ(−0), there is also a condition on the wave-function derivative (which experiences a jump): ψ 0 (+0) − ψ 0 (−0 ...
... decays at infinity.) Write down the normalization condition. (b) Impose the correct boundary conditions at the origin. Show that in addition to the wavefunction continuity equation ψ(+0) = ψ(−0), there is also a condition on the wave-function derivative (which experiences a jump): ψ 0 (+0) − ψ 0 (−0 ...
Chapter 4 - Mr. Fischer.com
... An atom is the smallest particle of an element that retains its identity in a chemical reaction. A. Early philosophers believed that atoms were indivisible and indestructible. B. Dalton’s Atomic theory. Dalton used experimental methods, to transform Democritus’s ideas on atoms into scientific theory ...
... An atom is the smallest particle of an element that retains its identity in a chemical reaction. A. Early philosophers believed that atoms were indivisible and indestructible. B. Dalton’s Atomic theory. Dalton used experimental methods, to transform Democritus’s ideas on atoms into scientific theory ...
CHAPTER 8 PERIODIC RELATIONSHIPS AMONG THE ELEMENTS
... table and it decreases down a group (column). Of the choices, K will have the smallest ionization energy. Ca, just to the right of K, will have a higher first ionization energy. Moving to the right across the periodic table, the ionization energies will continue to increase as we move to P. Continui ...
... table and it decreases down a group (column). Of the choices, K will have the smallest ionization energy. Ca, just to the right of K, will have a higher first ionization energy. Moving to the right across the periodic table, the ionization energies will continue to increase as we move to P. Continui ...
Balmer Series
... Universe is made of hydrogen. Emission or absorption processes in the hydrogen atom give rise to several line series, which are sequences of lines corresponding to electron transitions, each ending or beginning with the same atomic state in hydrogen. Thus, for example, the Balmer Series involves tra ...
... Universe is made of hydrogen. Emission or absorption processes in the hydrogen atom give rise to several line series, which are sequences of lines corresponding to electron transitions, each ending or beginning with the same atomic state in hydrogen. Thus, for example, the Balmer Series involves tra ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.