Bohr Model of the Atom
... The Danish physicist Niels Bohr, who first presented this model of the atom, based it on 3 fundamental postulates. (1) Electrons move around the nucleus in circular non-radiating orbits - called “stationary states”. However, they are not at rest! (2) An atom only emits or absorbs electromagnetic rad ...
... The Danish physicist Niels Bohr, who first presented this model of the atom, based it on 3 fundamental postulates. (1) Electrons move around the nucleus in circular non-radiating orbits - called “stationary states”. However, they are not at rest! (2) An atom only emits or absorbs electromagnetic rad ...
Modern physics
... • Bohr quantized angular momentum, for better H atom model. • Bohr model explained observed H spectra, derived En = E/n2 and phenomenological Rydberg constant • Quantum numbers n, l, ml (Zeeman effect) • Solution to Schrodinger equation showed that En = E/l(l+1) • Pauli proposed spin (ms=1/2), and ...
... • Bohr quantized angular momentum, for better H atom model. • Bohr model explained observed H spectra, derived En = E/n2 and phenomenological Rydberg constant • Quantum numbers n, l, ml (Zeeman effect) • Solution to Schrodinger equation showed that En = E/l(l+1) • Pauli proposed spin (ms=1/2), and ...
General Chemistry - Review for final exam: (Make sure you bring
... 30. How many orbitals & electrons are in the following systems? a. s b. p c. d d. f 31. What is the max # of electrons that can fit into an orbital? ...
... 30. How many orbitals & electrons are in the following systems? a. s b. p c. d d. f 31. What is the max # of electrons that can fit into an orbital? ...
worksheet 7b answers - Iowa State University
... Iowa State University 1) Effective Nuclear Charge: the net positive charge experienced by an electron in a many-electron atom. What is the equation? Zeff = Z – S Z = atoms number (# of protons or electrons) S = Shielding/Screening electrons Same n: 0.35 n-1: 0.85 n-2,3+: 1 ...
... Iowa State University 1) Effective Nuclear Charge: the net positive charge experienced by an electron in a many-electron atom. What is the equation? Zeff = Z – S Z = atoms number (# of protons or electrons) S = Shielding/Screening electrons Same n: 0.35 n-1: 0.85 n-2,3+: 1 ...
Document
... • 1926 – Enrico Fermi & Paul Dirac – formulated (independently) the Fermi-Dirac statistics, which describes distribution of many identical particles obeying the Pauli exclusion principle (fermions with half-integer spins – contrary to bosons satisfying the Bose-Einstein statistics) • 1926 – Erwin Sc ...
... • 1926 – Enrico Fermi & Paul Dirac – formulated (independently) the Fermi-Dirac statistics, which describes distribution of many identical particles obeying the Pauli exclusion principle (fermions with half-integer spins – contrary to bosons satisfying the Bose-Einstein statistics) • 1926 – Erwin Sc ...
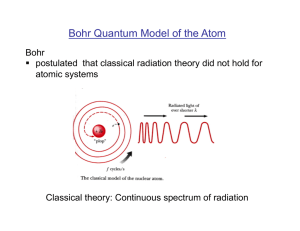
“solar system” model of the atom
... Experiments were done to confirm this model, looking at how alpha particles (helium nuclei) scattered from a gold foil. They found many more large-angle scatters than expected – something that could only happen if the positive charge were concentrated in a tiny volume, rather than spread throughout ...
... Experiments were done to confirm this model, looking at how alpha particles (helium nuclei) scattered from a gold foil. They found many more large-angle scatters than expected – something that could only happen if the positive charge were concentrated in a tiny volume, rather than spread throughout ...
Bohr Quantum Model of the Atom
... § postulated that the electron orbital momentum is quantized Justification of Bohr’s postulates: comparison with experimental observations! ...
... § postulated that the electron orbital momentum is quantized Justification of Bohr’s postulates: comparison with experimental observations! ...
Chap 7 - HCC Learning Web
... electrons are in the same group and thus will have similar chemical properties (see chapter 2). Another method is by adding the electrons altogether to get the total electron number which is same as the atomic number or say proton number in an atom. Then locate its symbol and location in the periodi ...
... electrons are in the same group and thus will have similar chemical properties (see chapter 2). Another method is by adding the electrons altogether to get the total electron number which is same as the atomic number or say proton number in an atom. Then locate its symbol and location in the periodi ...
Set #4 - comsics
... electron typically spends about 10-8 s in an excited state before it drops to a lower state by emitting a photon. How many revolutions does an electron in an n = 2 Bohr orbit make in 10-8 s? ...
... electron typically spends about 10-8 s in an excited state before it drops to a lower state by emitting a photon. How many revolutions does an electron in an n = 2 Bohr orbit make in 10-8 s? ...
Document
... Belief: Attractive force between the positively charged nucleus and an electron orbiting around is equal to the centrifugal exerted on the electron. This balance determines the electron’s radius. Challenge: A force is exerted on the electron, then, the electron should accelerate continuously accordi ...
... Belief: Attractive force between the positively charged nucleus and an electron orbiting around is equal to the centrifugal exerted on the electron. This balance determines the electron’s radius. Challenge: A force is exerted on the electron, then, the electron should accelerate continuously accordi ...
homework answers - SPHS Devil Physics
... a. Describe emission and absorption spectra and understand their significance for atomic structure b. Explain the origin of atomic energy levels in terms of the ‘electron in a box’ model c. Describe the hydrogen atom according to Schrödinger d. Do calculations involving wavelengths of spectral lines ...
... a. Describe emission and absorption spectra and understand their significance for atomic structure b. Explain the origin of atomic energy levels in terms of the ‘electron in a box’ model c. Describe the hydrogen atom according to Schrödinger d. Do calculations involving wavelengths of spectral lines ...
Chapter2. Elements of quantum mechanics
... An observation by Plank : radiation from a heated sample is emitted in discrete units of energy, called quanta ; the energy units were described by h, where is the frequency of the radiation, and h is a quantity called Plank’s constant En=nhν=nћω, h = 6.63 × 10-34 J·s, ћ=h/2π Quantization of ligh ...
... An observation by Plank : radiation from a heated sample is emitted in discrete units of energy, called quanta ; the energy units were described by h, where is the frequency of the radiation, and h is a quantity called Plank’s constant En=nhν=nћω, h = 6.63 × 10-34 J·s, ћ=h/2π Quantization of ligh ...
Lecture 4
... 3. If it is degenerate, how many states have the same energy and what are their quantum numbers ? (ignore spin) Answers ...
... 3. If it is degenerate, how many states have the same energy and what are their quantum numbers ? (ignore spin) Answers ...
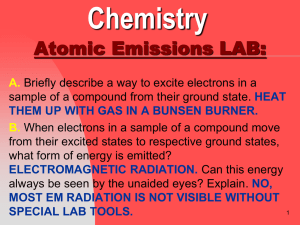
Atomic Emissions LAB Questions
... EACH ELEMENT HAS A UNIQUE SET OF SPECTAL LINES (IS LIKE A FINGER PRINT). F. Why is it possible for a sample of the element hydrogen, in which each atom only has one electron, to have an emission spectrum with more than one color of light? A SAMPLE HAS MANY ATOMS; EACH ELECTRON IN EACH ATOM WILL MOVE ...
... EACH ELEMENT HAS A UNIQUE SET OF SPECTAL LINES (IS LIKE A FINGER PRINT). F. Why is it possible for a sample of the element hydrogen, in which each atom only has one electron, to have an emission spectrum with more than one color of light? A SAMPLE HAS MANY ATOMS; EACH ELECTRON IN EACH ATOM WILL MOVE ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.