Learning Standards vocab chemical basis and molecules of life 09
... Given the number of protons, identify the element using a Periodic Table. Explain the arrangement of the elements on the Periodic Table, including the significant relationships among elements in a given column or row. Explain how ions and ionic bonds are formed (e.g., sodium atoms lose an elec ...
... Given the number of protons, identify the element using a Periodic Table. Explain the arrangement of the elements on the Periodic Table, including the significant relationships among elements in a given column or row. Explain how ions and ionic bonds are formed (e.g., sodium atoms lose an elec ...
Chapter 8 (Lecture 11) Atomic Orbitals The energy depends on the
... The closest shell to the nucleus is called the "1 shell" (also called "K shell"), followed by the "2 shell" (or "L shell"), then the "3 shell" (or "M shell"), and so on farther and farther from the nucleus. The shells correspond with the principal quantum numbers (1, 2, 3, 4 ...) or are labeled alp ...
... The closest shell to the nucleus is called the "1 shell" (also called "K shell"), followed by the "2 shell" (or "L shell"), then the "3 shell" (or "M shell"), and so on farther and farther from the nucleus. The shells correspond with the principal quantum numbers (1, 2, 3, 4 ...) or are labeled alp ...
The end
... respectively. Find the numerical threshold wavelength for the photoelectric effect of the cathode. b/ One milliwatt of light of wavelength 4,560A is incident on a caesium surface. Calculate the electron current liberated and the minimum stopping voltage necessary to reduce this current to zero. Work ...
... respectively. Find the numerical threshold wavelength for the photoelectric effect of the cathode. b/ One milliwatt of light of wavelength 4,560A is incident on a caesium surface. Calculate the electron current liberated and the minimum stopping voltage necessary to reduce this current to zero. Work ...
electron configuration
... 1. Aufbau Principle: electrons enter the lowest energy state possible 2. Pauli Exclusion Principle: at most 2 electrons per orbital; these must have opposite spins 3. Hund’s Rule: electrons do not pair until each orbital in a sublevel has at least 1 electron ...
... 1. Aufbau Principle: electrons enter the lowest energy state possible 2. Pauli Exclusion Principle: at most 2 electrons per orbital; these must have opposite spins 3. Hund’s Rule: electrons do not pair until each orbital in a sublevel has at least 1 electron ...
Physical Chemistry The hydrogen atom Center of mass
... The spatial part is incomplete One incorporates spin as a separate coordinate Wave function is a product n ,l ,m , I ,mI (r , , ) ...
... The spatial part is incomplete One incorporates spin as a separate coordinate Wave function is a product n ,l ,m , I ,mI (r , , ) ...
Quantum Mechanics OK
... • Erwin Schrödinger developed a mathematical treatment into which both the wave and particle nature of matter could be incorporated. • It is known as quantum mechanics. ...
... • Erwin Schrödinger developed a mathematical treatment into which both the wave and particle nature of matter could be incorporated. • It is known as quantum mechanics. ...
Adobe Acrobat file () - Wayne State University Physics and
... electrons, when an electron moves from the n = 1 level to the n = 3 level, the circumference of its orbit becomes 9 times greater. This occurs because (a) there are 3 times as many wavelengths in the new orbit, (b) there are 3 times as many wavelengths and each wavelength is 3 times as long, (c) the ...
... electrons, when an electron moves from the n = 1 level to the n = 3 level, the circumference of its orbit becomes 9 times greater. This occurs because (a) there are 3 times as many wavelengths in the new orbit, (b) there are 3 times as many wavelengths and each wavelength is 3 times as long, (c) the ...
Document
... for electrons in an atom. • The principal quantum number (n) describes the size and energy of the electron orbital. • Sublevels (l) describe the shape of orbitals. The number of sublevels = n • The direction (m) describes orientation of the sublevels. • Spin (s) refers to how an electron ...
... for electrons in an atom. • The principal quantum number (n) describes the size and energy of the electron orbital. • Sublevels (l) describe the shape of orbitals. The number of sublevels = n • The direction (m) describes orientation of the sublevels. • Spin (s) refers to how an electron ...
2nd Semester Review
... 4. Circle the correct atomic particle for each of the following: Defines an atom Protons Neutrons Electrons Isotopes: same type of atom with different number of Protons Neutrons Determines how atoms combine Protons Neutrons Electrons Ions: same type of atom with different number of Protons Neutrons ...
... 4. Circle the correct atomic particle for each of the following: Defines an atom Protons Neutrons Electrons Isotopes: same type of atom with different number of Protons Neutrons Determines how atoms combine Protons Neutrons Electrons Ions: same type of atom with different number of Protons Neutrons ...
Objective 6: TSW explain how the quantum
... • The orbits proposed by Bohr were considered electron waves and the electron wave characteristics were directly related to the probability of the location of an electron • The location of an electron was represented as a cloud (hence the reason the quantum mechanical model is sometimes referred to ...
... • The orbits proposed by Bohr were considered electron waves and the electron wave characteristics were directly related to the probability of the location of an electron • The location of an electron was represented as a cloud (hence the reason the quantum mechanical model is sometimes referred to ...
quantum numbers - misshoughton.net
... 4 quantum numbers apply equally for the electron orbits (paths), as well as, electron orbitals (clouds) [see Fig. 1 & Table 2 on p. 185] 1st 2 quantum numbers (n & l) describe electrons that have different energies under normal circumstances in multi-electron atoms the last 2 quantum numbers d ...
... 4 quantum numbers apply equally for the electron orbits (paths), as well as, electron orbitals (clouds) [see Fig. 1 & Table 2 on p. 185] 1st 2 quantum numbers (n & l) describe electrons that have different energies under normal circumstances in multi-electron atoms the last 2 quantum numbers d ...
Quantum mechanics in electronics
... 1. QUANTUM TUNNELLING In short an electron wave with less potential crosses a barrier with larger potential(explain) ...
... 1. QUANTUM TUNNELLING In short an electron wave with less potential crosses a barrier with larger potential(explain) ...
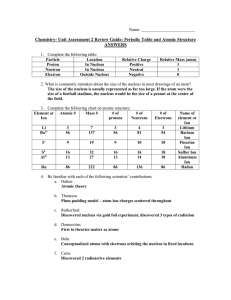
Chem Unit 2 Review Guide ANSWERS
... number (protons), but different masses because Hydrogen-2 has one neutron, while Hydrogen-3 has two neutrons. 8. Rubidium has two common isotopes, Rb-85 and Rb-87. If the atomic mass of Rubidium is 85.56 amu, what isotope is more abundant? Explain. Rb-85 is more abundant. If the amounts were equal, ...
... number (protons), but different masses because Hydrogen-2 has one neutron, while Hydrogen-3 has two neutrons. 8. Rubidium has two common isotopes, Rb-85 and Rb-87. If the atomic mass of Rubidium is 85.56 amu, what isotope is more abundant? Explain. Rb-85 is more abundant. If the amounts were equal, ...
4.2 Notes - Seymour ISD
... indicates the orientation of an orbital around the nucleus. • The spin quantum number has only two possible values—(+1/2 , −1/2)—which indicate the two fundamental spin states of an electron in an orbital. ...
... indicates the orientation of an orbital around the nucleus. • The spin quantum number has only two possible values—(+1/2 , −1/2)—which indicate the two fundamental spin states of an electron in an orbital. ...
(2 hours) This paper con - University of Southampton
... Ĥ , give the integral which is the expectation value of the energy for a one dimensional system extending from negative to positive infinity in the x direction. If ψ1 , ψ2 and ψ3 are orthonormal eigenfunctions of the Hamiltonian with energies 2 eV, 3 eV and 12 eV respectively, compute the expectati ...
... Ĥ , give the integral which is the expectation value of the energy for a one dimensional system extending from negative to positive infinity in the x direction. If ψ1 , ψ2 and ψ3 are orthonormal eigenfunctions of the Hamiltonian with energies 2 eV, 3 eV and 12 eV respectively, compute the expectati ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.