Quantum Numbers
... • Aufbau principle – electrons fill energy levels and sublevels in order of increasing energy • Pauli Exclusion principle – no two electrons can have the same set of four quantum numbers (which means no two electrons can be in the same place at the same time) • Hund’s rule – when adding electrons to ...
... • Aufbau principle – electrons fill energy levels and sublevels in order of increasing energy • Pauli Exclusion principle – no two electrons can have the same set of four quantum numbers (which means no two electrons can be in the same place at the same time) • Hund’s rule – when adding electrons to ...
Topic 2 - Jensen Chemistry
... In the following pictures, there is a target hidden by a cloud. To figure out the shape of the target, we shot some beams into the cloud and recorded where the beams came out. Can you figure out the shape of the ...
... In the following pictures, there is a target hidden by a cloud. To figure out the shape of the target, we shot some beams into the cloud and recorded where the beams came out. Can you figure out the shape of the ...
Quantum Mechanics
... they can teach the professors, and Feynman was one of the best (students). ...
... they can teach the professors, and Feynman was one of the best (students). ...
n = 2. - Cloudfront.net
... shapes than spherical as the possible values of the orbital quantum number increase. ...
... shapes than spherical as the possible values of the orbital quantum number increase. ...
Lecture notes 2: Quantum mechanics in a nutshell
... can be built up by neglecting electron spin (but do keep the exclusion principle). This means any electron configuration can have two electrons — one with spin up, the other with spin down. Assume further that each electron moves in a central field which is the combined field of the nucleus and all ...
... can be built up by neglecting electron spin (but do keep the exclusion principle). This means any electron configuration can have two electrons — one with spin up, the other with spin down. Assume further that each electron moves in a central field which is the combined field of the nucleus and all ...
atomic theory - unit a
... 1) n = principal quantum number, where n is energy shell. Values for n = 1,2,3,4... (n = 1 closest shell to nucleus) Generally, energy increases with increasing n. 2) Principle energy levels can be subdivided into subshells. • Electrons within a subshell have identical energy. • There are 4 known su ...
... 1) n = principal quantum number, where n is energy shell. Values for n = 1,2,3,4... (n = 1 closest shell to nucleus) Generally, energy increases with increasing n. 2) Principle energy levels can be subdivided into subshells. • Electrons within a subshell have identical energy. • There are 4 known su ...
Electronic Structure of Atoms
... Bohr noted the line spectra of certain elements and assumed that electrons were confined to specific energy states. These were called orbits. Bohr’s model is based on three postulates: • Only orbits of specific radii, corresponding to certain definite energies, are permitted for electrons in an atom ...
... Bohr noted the line spectra of certain elements and assumed that electrons were confined to specific energy states. These were called orbits. Bohr’s model is based on three postulates: • Only orbits of specific radii, corresponding to certain definite energies, are permitted for electrons in an atom ...
Structure of Atom Easy Notes
... Classical mechanics is based on Newton’s laws of motion. It successfully describes the motion of macroscopic particles but fails in the case of microscopic particles. Reason: Classical mechanics ignores the concept of dual behaviour of matter especially for subatomic particles and the Heisenberg’s u ...
... Classical mechanics is based on Newton’s laws of motion. It successfully describes the motion of macroscopic particles but fails in the case of microscopic particles. Reason: Classical mechanics ignores the concept of dual behaviour of matter especially for subatomic particles and the Heisenberg’s u ...
QUANTUM THEORY OF ATOMS AND MOLECULES
... energy from the ground to the first excited vibrational states. 3. Calculate the difference between the zero-point energy (in kJ mol) of C-H and C-D bond stretches, given a C-H vibrational stretching frequency of 2900 cm. Hence explain why C-H bonds react more rapidly than C-D bonds in many orga ...
... energy from the ground to the first excited vibrational states. 3. Calculate the difference between the zero-point energy (in kJ mol) of C-H and C-D bond stretches, given a C-H vibrational stretching frequency of 2900 cm. Hence explain why C-H bonds react more rapidly than C-D bonds in many orga ...
power point notes
... B. Dalton’s Atomic Theory (1808) Stated that elements are made up of tiny particles called Atoms and each chemical has a unique combination of atoms ...
... B. Dalton’s Atomic Theory (1808) Stated that elements are made up of tiny particles called Atoms and each chemical has a unique combination of atoms ...
PPT - kimscience.com
... nucleus-attracted to the protons When one energy level is filled, electrons are found at higher levels. Each energy level can hold a maximum number of electrons (2n2 electrons) First shell = two electrons Second shell = eight electrons Third shell = eighteen electrons ...
... nucleus-attracted to the protons When one energy level is filled, electrons are found at higher levels. Each energy level can hold a maximum number of electrons (2n2 electrons) First shell = two electrons Second shell = eight electrons Third shell = eighteen electrons ...
Q: In which model of the atom do electrons orbit the nucleus? A
... of what block on the periodic table? A: p block ...
... of what block on the periodic table? A: p block ...
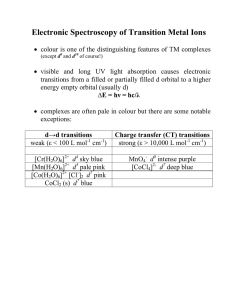
Electronic Spectroscopy of Transition Metal Ions
... With most TM ions, the spin-orbit coupling is small due to electron delocalization onto the ligands so the energy differences between the possible J states are negligible but the possibilities would be J = 4, 3 or 2 giving rise to 3F4, 3F3 and 3F2 states. ...
... With most TM ions, the spin-orbit coupling is small due to electron delocalization onto the ligands so the energy differences between the possible J states are negligible but the possibilities would be J = 4, 3 or 2 giving rise to 3F4, 3F3 and 3F2 states. ...
Abstract - Quantum Realism and Special Reference
... increases. One possible construal of the expression L = l l 1 then would be to have the radius of the orbital be proportional to n2 – ¼ and the rectilinear velocity of the orbital be proportional to 1/(n - ½) since ...
... increases. One possible construal of the expression L = l l 1 then would be to have the radius of the orbital be proportional to n2 – ¼ and the rectilinear velocity of the orbital be proportional to 1/(n - ½) since ...
AtomsFirst2e_day6_sec3.7
... The allowed values of n are integral numbers: 1, 2, 3 and so forth. The value of n corresponds to the value of n in Bohr’s model of the hydrogen atom. ...
... The allowed values of n are integral numbers: 1, 2, 3 and so forth. The value of n corresponds to the value of n in Bohr’s model of the hydrogen atom. ...
Orbital Hybridisation www.AssignmentPoint.com In chemistry
... chemical bonds in valence bond theory. Hybrid orbitals are very useful in the explanation of molecular geometry and atomic bonding properties. Although sometimes taught together with the valence shell electron-pair repulsion (VSEPR) theory, valence bond and hybridisation are in fact not related to t ...
... chemical bonds in valence bond theory. Hybrid orbitals are very useful in the explanation of molecular geometry and atomic bonding properties. Although sometimes taught together with the valence shell electron-pair repulsion (VSEPR) theory, valence bond and hybridisation are in fact not related to t ...
Electromagnetic Radiation
... 1.) The electron travels in orbits (energy levels) around the nucleus. 2.) The orbits closest to the nucleus are lowest in energy, those further out are higher in energy. 3.) When energy is absorbed by the atom, the electron moves into a higher energy orbit. This energy is released when the elec ...
... 1.) The electron travels in orbits (energy levels) around the nucleus. 2.) The orbits closest to the nucleus are lowest in energy, those further out are higher in energy. 3.) When energy is absorbed by the atom, the electron moves into a higher energy orbit. This energy is released when the elec ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.