Quantum Coherence between States with Even and Odd Numbers of Electrons
... In 1952, Wick, Wightman, and Wigner [1] claimed that the coherent linear superpositions of states with even and odd numbers of fermions are incompatible with the Lorentz invariance and introduced the superselection rule, according to which such linear superpositions are physically impossible. In act ...
... In 1952, Wick, Wightman, and Wigner [1] claimed that the coherent linear superpositions of states with even and odd numbers of fermions are incompatible with the Lorentz invariance and introduced the superselection rule, according to which such linear superpositions are physically impossible. In act ...
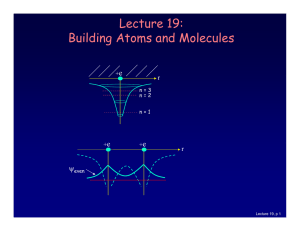
Lecture 19: Building Atoms and Molecules
... For atoms with many electrons (e.g., carbon: 6, iron: 26, etc.) 4 What energies do the electrons have? “Pauli Exclusion Principle” (1925) No two electrons can be in the same quantum state. For example, in a given atom they cannot have the same set of quantum numbers n, l, ml, ms. This means that eac ...
... For atoms with many electrons (e.g., carbon: 6, iron: 26, etc.) 4 What energies do the electrons have? “Pauli Exclusion Principle” (1925) No two electrons can be in the same quantum state. For example, in a given atom they cannot have the same set of quantum numbers n, l, ml, ms. This means that eac ...
Document
... Electrons as Waves in Three Dimensions The wavefunctions that describe electrons are three-dimensional waves. They have similar properties and features as one- and twodimensional waves. i.e. Positive and negative lobes, and nodes (which are planes in 3-D). The quantum description of an electron is ...
... Electrons as Waves in Three Dimensions The wavefunctions that describe electrons are three-dimensional waves. They have similar properties and features as one- and twodimensional waves. i.e. Positive and negative lobes, and nodes (which are planes in 3-D). The quantum description of an electron is ...
Hybridization and St..
... Hybridization also occurs in compounds of beryllium. The electron configuration if Be is 1s22s2. It would appear to have no half-filled orbitals with which to form covalent bonds. ...
... Hybridization also occurs in compounds of beryllium. The electron configuration if Be is 1s22s2. It would appear to have no half-filled orbitals with which to form covalent bonds. ...
Chemistry Study Guide
... Chemistry Study Guide Atoms and Subatomic Particles Atom. Al matter is made up of unique particles called atoms. An atom contains: o Protons- Positively charged; have atomic mass of one; Located in the nucleus of the atom. o Neutrons- Neutral in charge; the same mass as the proton; also located in ...
... Chemistry Study Guide Atoms and Subatomic Particles Atom. Al matter is made up of unique particles called atoms. An atom contains: o Protons- Positively charged; have atomic mass of one; Located in the nucleus of the atom. o Neutrons- Neutral in charge; the same mass as the proton; also located in ...
wave-particle duality
... We cannot see the wave/particle nature at the same time. If we know which path the particle takes, we lose the fringes . ...
... We cannot see the wave/particle nature at the same time. If we know which path the particle takes, we lose the fringes . ...
WAVE-PARTICLE DUALITY
... We cannot see the wave/particle nature at the same time. If we know which path the particle takes, we lose the fringes . ...
... We cannot see the wave/particle nature at the same time. If we know which path the particle takes, we lose the fringes . ...
Artificial atoms
... In a natural atom one has little control over the spectrum of energies for adding or removing electrons. There the electrons interact with the fixed potential of the nucleus and with each other, and these two kinds of interaction determine the spectrum. In an artificial atom, however, one can change ...
... In a natural atom one has little control over the spectrum of energies for adding or removing electrons. There the electrons interact with the fixed potential of the nucleus and with each other, and these two kinds of interaction determine the spectrum. In an artificial atom, however, one can change ...
Molecular orbital methods in organic chemistry
... implicitly built in. Molecular orbital theories satisfy this type of condition insofar as each electron is treated as being free to move in a path covering the entire molecular framework. A further requirement for a useful theory is that the approximate wave functions used must be amenable to detail ...
... implicitly built in. Molecular orbital theories satisfy this type of condition insofar as each electron is treated as being free to move in a path covering the entire molecular framework. A further requirement for a useful theory is that the approximate wave functions used must be amenable to detail ...
Atom Models Timeline
... 3. Be chronological in its sequence, with the dates clearly shown (it need not be to scale), and at a minimum include the information contained in the following tables. 4. Be neat and legible. Deadlines and Guidelines: Your timeline is due on ____________________. 15 points will be deducted for ev ...
... 3. Be chronological in its sequence, with the dates clearly shown (it need not be to scale), and at a minimum include the information contained in the following tables. 4. Be neat and legible. Deadlines and Guidelines: Your timeline is due on ____________________. 15 points will be deducted for ev ...
Quantum Chemistry
... 3. The Rutherford picture of an atom with electrons orbiting around a central atom is inconsistent with the laws of classical physics. Unlike planets orbiting around a star, an orbiting electron is a moving charge and should radiate energy as it spirals towards the nucleus. Neils Bohr, who had been ...
... 3. The Rutherford picture of an atom with electrons orbiting around a central atom is inconsistent with the laws of classical physics. Unlike planets orbiting around a star, an orbiting electron is a moving charge and should radiate energy as it spirals towards the nucleus. Neils Bohr, who had been ...
Quantum Mechanics
... 4. A particle moves in one dimension and in a potential of the form V (x) = 0, for |x| < a and V (x) = V0 > 0 for |x| > a. The particle has energy 0 < E < V0 . a. Solve the Schrödinger equation in each of the three regions: I: −∞ < x < −a, II: −a < x < +a and III: +a < x < +∞. b. Specify the contin ...
... 4. A particle moves in one dimension and in a potential of the form V (x) = 0, for |x| < a and V (x) = V0 > 0 for |x| > a. The particle has energy 0 < E < V0 . a. Solve the Schrödinger equation in each of the three regions: I: −∞ < x < −a, II: −a < x < +a and III: +a < x < +∞. b. Specify the contin ...
Ch.4-Electron Arrangement in Atoms
... exist in defined orbits, but in regions around the nucleus. The regions around the nucleus came to be known as orbitals. Orbitals are three-dimensional regions around the nucleus that indicate the probable location of an electron. ...
... exist in defined orbits, but in regions around the nucleus. The regions around the nucleus came to be known as orbitals. Orbitals are three-dimensional regions around the nucleus that indicate the probable location of an electron. ...
Chapter 28
... Predicts a value for RH that agrees with the experimental value Gives an expression for the radius of the atom Predicts energy levels of hydrogen Gives a model of what the atom looks like and ...
... Predicts a value for RH that agrees with the experimental value Gives an expression for the radius of the atom Predicts energy levels of hydrogen Gives a model of what the atom looks like and ...
Slide 1
... •The relationship between these levels of organization depends upon the energy state of the atom, which in turn depends upon a quantity called the principle quantum number. •Shell: Identified by a principal quantum number (1, 2, 3 . . . n) that specifies the energy level, or energy state, of the she ...
... •The relationship between these levels of organization depends upon the energy state of the atom, which in turn depends upon a quantity called the principle quantum number. •Shell: Identified by a principal quantum number (1, 2, 3 . . . n) that specifies the energy level, or energy state, of the she ...
Chemistry Honors Unit 2 Study Guide Atomic Theory Mr. Brown Use
... Joseph John Thomson- He discovered the electron and established that they were a part of all atoms. He measured the charge to mass ratio of the electron. He came up with the plum pudding model of the atom in which the electrons were thought to be evenly distributed throughout the atom as raisins are ...
... Joseph John Thomson- He discovered the electron and established that they were a part of all atoms. He measured the charge to mass ratio of the electron. He came up with the plum pudding model of the atom in which the electrons were thought to be evenly distributed throughout the atom as raisins are ...
Orbitals and Quantum Numbers
... an electron in the quantum-mechanical model of the atom the term orbital is also used to describe the spatial distribution of the electron. ...
... an electron in the quantum-mechanical model of the atom the term orbital is also used to describe the spatial distribution of the electron. ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.