Document
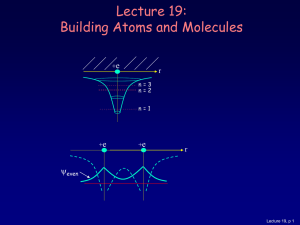
... experiment, the electrons must have the same wavelength To have the same wavelength, the electrons must have the same momentum or equivalently, energy ...
... experiment, the electrons must have the same wavelength To have the same wavelength, the electrons must have the same momentum or equivalently, energy ...
lecture25
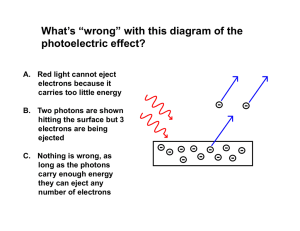
... If light is particles, theory predicts: • Increasing intensity increases number of electrons but not energy • Above a minimum energy required to break atomic bond, kinetic energy will increase linearly with frequency • There is a cutoff frequency below which no electrons will be emitted, regardless ...
... If light is particles, theory predicts: • Increasing intensity increases number of electrons but not energy • Above a minimum energy required to break atomic bond, kinetic energy will increase linearly with frequency • There is a cutoff frequency below which no electrons will be emitted, regardless ...
Chapter 1 - Atoms: The Quantum World
... catastrophe, quanta, Planck constant, photoelectric effect, photons, work function, diffraction, constructive and destructive interference, duality of electromagnetic radiation and matter, linear momentum, de Broglie relation, Heisenberg uncertainty principle (complementarity of location and momentu ...
... catastrophe, quanta, Planck constant, photoelectric effect, photons, work function, diffraction, constructive and destructive interference, duality of electromagnetic radiation and matter, linear momentum, de Broglie relation, Heisenberg uncertainty principle (complementarity of location and momentu ...
Assignment 3 - SOLUTIONS
... v = h/m λ = 6.626 × 10-34 Js/(1.0 × 102 g × 5.6 × 10-3 nm) = 6.626 × 10-34 Js/(0.1 kg × 5.6 × 10-12 m) = 1.2 × 10-21 Js kg-1 m-1 = 1.2 × 10-21 kg m2 s-2 s kg-1 m-1 = 1.2 × 10-21 m s-1 6. A possible excited state for the H atom has an electron in a 5d orbital. List all possible sets of quantum number ...
... v = h/m λ = 6.626 × 10-34 Js/(1.0 × 102 g × 5.6 × 10-3 nm) = 6.626 × 10-34 Js/(0.1 kg × 5.6 × 10-12 m) = 1.2 × 10-21 Js kg-1 m-1 = 1.2 × 10-21 kg m2 s-2 s kg-1 m-1 = 1.2 × 10-21 m s-1 6. A possible excited state for the H atom has an electron in a 5d orbital. List all possible sets of quantum number ...
Document
... Chap 6: Quantum Mechanics in One Dimension The Born interpretation, the Schrodinger equation, potential wells ...
... Chap 6: Quantum Mechanics in One Dimension The Born interpretation, the Schrodinger equation, potential wells ...
Chapter 2: You must understand chemistry to understand life (and to
... Overview: In many ways, life can be viewed as a complicated chemical reaction. Modern models of how life works at all levels typically have at least some aspect of chemistry as a major component or underpinning. I. ...
... Overview: In many ways, life can be viewed as a complicated chemical reaction. Modern models of how life works at all levels typically have at least some aspect of chemistry as a major component or underpinning. I. ...
electron
... How much radiation pressure is exerted by sunlight? Could the pressure of Sunlight be harnessed to “sail” across the solar system? If we know for example the amount of energy emitted by the Sun (1300 W/m2 at Earth), and the “average wavelength” of sunlight (500 nm)…. Then we can calculate the number ...
... How much radiation pressure is exerted by sunlight? Could the pressure of Sunlight be harnessed to “sail” across the solar system? If we know for example the amount of energy emitted by the Sun (1300 W/m2 at Earth), and the “average wavelength” of sunlight (500 nm)…. Then we can calculate the number ...
A = 27
... #33 The excited state must have the same # of electrons as the neutral atom, however one or more must be at a higher energy level (outermost shell) that the ground state of the periodic table ( for Al it is 2-8-3), 13 electrons.The ans is 1) 2-7-4 (13 e-), one electron promoted from shell 2 to shell ...
... #33 The excited state must have the same # of electrons as the neutral atom, however one or more must be at a higher energy level (outermost shell) that the ground state of the periodic table ( for Al it is 2-8-3), 13 electrons.The ans is 1) 2-7-4 (13 e-), one electron promoted from shell 2 to shell ...
Chapter 4 Notes - Atomic Theory
... Multivalent: some transition metals have more than one charge. Roman numerals are used after the metal name to indicate which ion was used Ex. 1 What is the formula manganese(III) sulphide? This manganese is Mn3+ Sulphur is S2– Lowest common multiple of 3 and 2 is 6 2 Mn3+ ions and 3 S2– i ...
... Multivalent: some transition metals have more than one charge. Roman numerals are used after the metal name to indicate which ion was used Ex. 1 What is the formula manganese(III) sulphide? This manganese is Mn3+ Sulphur is S2– Lowest common multiple of 3 and 2 is 6 2 Mn3+ ions and 3 S2– i ...
general properties of the solution: quantum numbers:
... - If L was aligned with the quantization axis the electron would be certain to move in the x-y plane. The uncertainty principle would require the momentum uncertainty Δpz to be infinite. Thus the electron could not be bound to the nucleus. - the uncertainty principle enforces that not all components ...
... - If L was aligned with the quantization axis the electron would be certain to move in the x-y plane. The uncertainty principle would require the momentum uncertainty Δpz to be infinite. Thus the electron could not be bound to the nucleus. - the uncertainty principle enforces that not all components ...
chapter2 2012 (no naming)
... • Rays are composed of negatively charged particles called electrons • Electrons carry unit negative charge (-1) and have a very small mass (1/2000 the lightest atomic mass) ...
... • Rays are composed of negatively charged particles called electrons • Electrons carry unit negative charge (-1) and have a very small mass (1/2000 the lightest atomic mass) ...
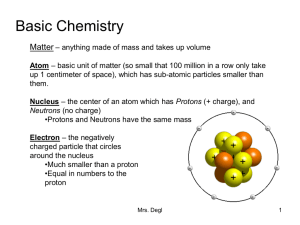
Basic Chemistry
... Isotopes – Atoms of the same element can have a different number of neutrons. Some Carbon atoms have 6 neutrons and some have 7/ 8. •Some isotopes can be radioactive (unstable nuclei that breaks down at a constant rate over time). Compound – when two or more elements are chemically combined •Bonds ...
... Isotopes – Atoms of the same element can have a different number of neutrons. Some Carbon atoms have 6 neutrons and some have 7/ 8. •Some isotopes can be radioactive (unstable nuclei that breaks down at a constant rate over time). Compound – when two or more elements are chemically combined •Bonds ...
Physics 361 Principles of Modern Physics
... For the atoms in the third and fourth group – they contain at least one empty p orbital. For such atoms, it does not cost much energy to take an electron in an s orbital and move it to the vacant p orbital. The physical reason for this is that the two s electrons will be feeling a relatively strong ...
... For the atoms in the third and fourth group – they contain at least one empty p orbital. For such atoms, it does not cost much energy to take an electron in an s orbital and move it to the vacant p orbital. The physical reason for this is that the two s electrons will be feeling a relatively strong ...
Electron Distribution Using Peas
... Could you determine the exact position and momentum of a baseball as it soared through the air? Of course, you could—by taking a timed series of snapshots of the baseball as it moved. Why then can’t scientists follow a similar procedure to determine the position and momentum of an electron? You can ...
... Could you determine the exact position and momentum of a baseball as it soared through the air? Of course, you could—by taking a timed series of snapshots of the baseball as it moved. Why then can’t scientists follow a similar procedure to determine the position and momentum of an electron? You can ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.