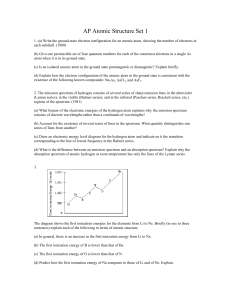
The “classically forbidden regions” are where … a. a particle`s total
... (takes more energy) • You can instead change their internal quantum numbers (if they have them). • Electrons do have 1 internal number that can be +1/2 or -1/2 so 2 of them can get into a state. ...
... (takes more energy) • You can instead change their internal quantum numbers (if they have them). • Electrons do have 1 internal number that can be +1/2 or -1/2 so 2 of them can get into a state. ...
CHE 1401 - Fall 2013 - Chapter 7 Homework 7 (Chapter 7: Periodic
... 12) Alkali metals tend to be more reactive than alkaline earth metals because __________. A) alkali metals have lower densities B) alkali metals have greater electron affinities C) alkali metals have lower ionization energies D) alkali metals have lower melting points E) alkali metals are not more r ...
... 12) Alkali metals tend to be more reactive than alkaline earth metals because __________. A) alkali metals have lower densities B) alkali metals have greater electron affinities C) alkali metals have lower ionization energies D) alkali metals have lower melting points E) alkali metals are not more r ...
Section 5.3 Physics and Quantum Mechanical Model
... through a diffraction grating, could you see the line corresponding to this emission? Which series of lines could human detect with our ...
... through a diffraction grating, could you see the line corresponding to this emission? Which series of lines could human detect with our ...
The Atom
... Elements are made of tiny particles called atoms All atoms of a given element are identical The atoms of a given element differ from those of other elements Atoms of one element can combine with those of other elements to form compounds, and a given compound always has the same relative numbers (rat ...
... Elements are made of tiny particles called atoms All atoms of a given element are identical The atoms of a given element differ from those of other elements Atoms of one element can combine with those of other elements to form compounds, and a given compound always has the same relative numbers (rat ...
Chemistry Curriculum Guide
... Ionization energies generally increase from left to right and decrease from top to bottom of a given group. ELECTRON CONFIGURATIONS, VALENCE ELECTRONS, AND OXIDATION NUMBERS ...
... Ionization energies generally increase from left to right and decrease from top to bottom of a given group. ELECTRON CONFIGURATIONS, VALENCE ELECTRONS, AND OXIDATION NUMBERS ...
AP Atomic Structure Set 1
... (b) Give one permissible set of four quantum numbers for each of the outermost electrons in a single As atom when it is in its ground state. (c) Is an isolated arsenic atom in the ground state paramagnetic or diamagnetic? Explain briefly. (d) Explain how the electron configuration of the arsenic ato ...
... (b) Give one permissible set of four quantum numbers for each of the outermost electrons in a single As atom when it is in its ground state. (c) Is an isolated arsenic atom in the ground state paramagnetic or diamagnetic? Explain briefly. (d) Explain how the electron configuration of the arsenic ato ...
Worksheet 1 Notes - Department of Chemistry | Oregon State
... Determine the electron configuration for O. Is O a reactive element? Why? Determine the electron configuration for O-. Is O- a stable ion? Why? Determine the electron configuration for O2-. Is O2- a stable ion? Why? O is 1s22s22p4. O is very reactive (the principal quantum numbers 1 and 2 energy lev ...
... Determine the electron configuration for O. Is O a reactive element? Why? Determine the electron configuration for O-. Is O- a stable ion? Why? Determine the electron configuration for O2-. Is O2- a stable ion? Why? O is 1s22s22p4. O is very reactive (the principal quantum numbers 1 and 2 energy lev ...
Physics 571 Lecture #27 - BYU Physics and Astronomy
... For two equivalent p electrons (equivalent because they are in the same n level), we have `1 = 1, `2 = 1, s1 = 12 , and s2 = 12 . So you can have L = 0, 1, 2 and S = 0, 1. Depending on each L and S value, you have a range of possible J values. But we need to be careful. Some of these combinations of ...
... For two equivalent p electrons (equivalent because they are in the same n level), we have `1 = 1, `2 = 1, s1 = 12 , and s2 = 12 . So you can have L = 0, 1, 2 and S = 0, 1. Depending on each L and S value, you have a range of possible J values. But we need to be careful. Some of these combinations of ...
Quantum Physics 3 - FSU Physics Department
... in the Swiss Alps for two weeks, taking with him his notebooks, two pearls, and an old Viennese girlfriend. Schrödinger's self-appointed mission was to save the patched-up, creaky quantum theory of the time. The Viennese physicist placed a pearl in each ear to screen out any distracting noises. Then ...
... in the Swiss Alps for two weeks, taking with him his notebooks, two pearls, and an old Viennese girlfriend. Schrödinger's self-appointed mission was to save the patched-up, creaky quantum theory of the time. The Viennese physicist placed a pearl in each ear to screen out any distracting noises. Then ...
SCH 3U - othsmath
... electrons so adding in another valence electron releases less (electron affinity) energy. 1) When reacting chemically, metals tend to lose one or more valence electrons to form positive ions. Going down a group, a new energy level is added with each subsequent atom, ensuring the valence electrons ar ...
... electrons so adding in another valence electron releases less (electron affinity) energy. 1) When reacting chemically, metals tend to lose one or more valence electrons to form positive ions. Going down a group, a new energy level is added with each subsequent atom, ensuring the valence electrons ar ...
Document
... For atoms with many electrons (e.g., carbon: 6, iron: 26, etc.) < What energies do the electrons have? “Pauli Exclusion Principle” (1925) No two electrons can be in the same quantum state. For example, in a given atom they cannot have the same set of quantum numbers n, l, ml, ms. This means that eac ...
... For atoms with many electrons (e.g., carbon: 6, iron: 26, etc.) < What energies do the electrons have? “Pauli Exclusion Principle” (1925) No two electrons can be in the same quantum state. For example, in a given atom they cannot have the same set of quantum numbers n, l, ml, ms. This means that eac ...
Student Text, pp. 650-653
... as a wave phenomenon, was apparently composed of photons possessing distinct particle characteristics. Electrons, to this point thought of as tiny particles with a definite charge and mass, behaved like waves with a definite wavelength, as they moved in orbits within the atom and interacted with obj ...
... as a wave phenomenon, was apparently composed of photons possessing distinct particle characteristics. Electrons, to this point thought of as tiny particles with a definite charge and mass, behaved like waves with a definite wavelength, as they moved in orbits within the atom and interacted with obj ...
Recitation on atomic structure Solution
... speed v, where v = V /Bd, while in the region where there is only a magnetic field the electron moves in a circle of radius r, with r given by p = Bre. This latter region (E = 0, B =constant) acts as a momentum selector because electrons with larger momenta have paths with larger radii. (a) Show that ...
... speed v, where v = V /Bd, while in the region where there is only a magnetic field the electron moves in a circle of radius r, with r given by p = Bre. This latter region (E = 0, B =constant) acts as a momentum selector because electrons with larger momenta have paths with larger radii. (a) Show that ...
Lecture 3 - Engineering
... + Eelectron spin (orientation of nuclear spin in a magnetic field) + Enuclear spin (orientation of electron spin in a magnetic field) + Erotation (rotation of the molecule about its center of mass) + Evibration (vibration of the molecule’s constituent atoms ) + Eelectronic (electronic transitions be ...
... + Eelectron spin (orientation of nuclear spin in a magnetic field) + Enuclear spin (orientation of electron spin in a magnetic field) + Erotation (rotation of the molecule about its center of mass) + Evibration (vibration of the molecule’s constituent atoms ) + Eelectronic (electronic transitions be ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.