Interaction of Photons with Matter
... The first spectrum was observed in the visible region for light emitted by hydrogen atoms: ...
... The first spectrum was observed in the visible region for light emitted by hydrogen atoms: ...
Atomic Structure, Eelectronic Bonding, Periodicity, orbitals
... – Theoretically, this series continues on to g,h,i, etc. orbitals. • Practically speaking atoms that have been discovered or made up to this point in time only have electrons in s, p, d, or f orbitals in their ground state configurations. • Each wave function with an allowed combination of n, l, and ...
... – Theoretically, this series continues on to g,h,i, etc. orbitals. • Practically speaking atoms that have been discovered or made up to this point in time only have electrons in s, p, d, or f orbitals in their ground state configurations. • Each wave function with an allowed combination of n, l, and ...
The Chemical Context of Life Chapter 2 Notes
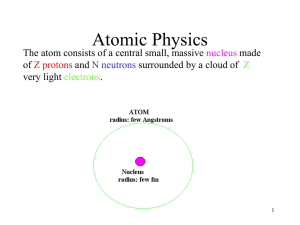
... Atomic number: # of protons Mass number: sum of protons + neutrons Isotopes: different atomic forms of an element. -ex. Carbon-12 (99%), Carbon-13 (1%), Carbon-14 (<1%) ...
... Atomic number: # of protons Mass number: sum of protons + neutrons Isotopes: different atomic forms of an element. -ex. Carbon-12 (99%), Carbon-13 (1%), Carbon-14 (<1%) ...
AtomLightEmissQuantum
... Some of hydrogen’s energy levels and the possible energy level transitions that it can undergo are shown in the figure at right. Note that an excited hydrogen atom can ...
... Some of hydrogen’s energy levels and the possible energy level transitions that it can undergo are shown in the figure at right. Note that an excited hydrogen atom can ...
GENERAL CHEMISTRY SECTION I: ATOMIC THEORY
... As expected, there is an inverse relationship between mass and wavelength. What are some examples of magnitudes in the relationship between λ and m? • An e- with mass of 10-31 kg can generate nm (nanometer; 10-9 m) waves • A proton with mass of 10-27 kg can generate pm (picometer; 10-12 m) waves • A ...
... As expected, there is an inverse relationship between mass and wavelength. What are some examples of magnitudes in the relationship between λ and m? • An e- with mass of 10-31 kg can generate nm (nanometer; 10-9 m) waves • A proton with mass of 10-27 kg can generate pm (picometer; 10-12 m) waves • A ...
Chapter 9
... Three (or more) atom molecules cannot be explained by simple overlap of orbitals. Fact: a bond generally forms between two half-filled orbitals. Fact: an s-type orbital is spherical, so it could form a bond in any direction. Fact: the three p-type orbitals are at 90 degree angles to each other. ...
... Three (or more) atom molecules cannot be explained by simple overlap of orbitals. Fact: a bond generally forms between two half-filled orbitals. Fact: an s-type orbital is spherical, so it could form a bond in any direction. Fact: the three p-type orbitals are at 90 degree angles to each other. ...
Section 1.5 - 1 1.5 The Vector Model of the Atom Classical Physics: If
... Obviously, j must be half-integral for a one-electron system, therefore j can be: j = (½ √3), (½ √15), (½ √35) by the formula given above for j; with j = ½, 3/2, 5/2, ... b) By summation of quantum numbers ml and ms (i.e. the possible values of the zcomponent of l and s). This method is generally ap ...
... Obviously, j must be half-integral for a one-electron system, therefore j can be: j = (½ √3), (½ √15), (½ √35) by the formula given above for j; with j = ½, 3/2, 5/2, ... b) By summation of quantum numbers ml and ms (i.e. the possible values of the zcomponent of l and s). This method is generally ap ...
Review for Test II
... B. Dalton (early 1800's) 1. Each element is composed of tiny indestructible particles called atoms 2. All atoms of a given element have the same mass and other properties that distinguish them from atoms of other elements 3. Atoms combine in simple, whole-number ratios to form compounds -Law of cons ...
... B. Dalton (early 1800's) 1. Each element is composed of tiny indestructible particles called atoms 2. All atoms of a given element have the same mass and other properties that distinguish them from atoms of other elements 3. Atoms combine in simple, whole-number ratios to form compounds -Law of cons ...
Conduction and Semiconductors
... Electron band diagrams are a way to visualize what happens at a p-n junction, using the following rules: 1. The Fermi level must be at the same level on both sides of the junction when there is no applied field 2. Far from the junctions, the materials inherent electrical structure exists 3. He band ...
... Electron band diagrams are a way to visualize what happens at a p-n junction, using the following rules: 1. The Fermi level must be at the same level on both sides of the junction when there is no applied field 2. Far from the junctions, the materials inherent electrical structure exists 3. He band ...
Summer Assignment 2015
... Name ________________________________ Complete this assignment on a separate sheet(s) of paper. To receive full credit you must show all work, report your answers to the proper number of significant figures, and your answers must have the appropriate units. Refer to the links on the last page for he ...
... Name ________________________________ Complete this assignment on a separate sheet(s) of paper. To receive full credit you must show all work, report your answers to the proper number of significant figures, and your answers must have the appropriate units. Refer to the links on the last page for he ...
A FERMI SEA OF HEAVY ELECTRONS
... which we must use to describe the wave function has radically changed. N sites on which we may have a spin up or down have 2N possible states; but if we can occupy each of these N sites with 0, 1 or 2 real electrons that amounts to 4N possible states. (If there is orbital degeneracy that merely chan ...
... which we must use to describe the wave function has radically changed. N sites on which we may have a spin up or down have 2N possible states; but if we can occupy each of these N sites with 0, 1 or 2 real electrons that amounts to 4N possible states. (If there is orbital degeneracy that merely chan ...
CHAPTER 6 PRACTICE TEST Name Relevant Equations E = hν E
... They tend to form molecular compounds in which electrons are shared with other atoms. Reactivity of these elements tends to decrease as atomic number increases. These elements tend to be more reactive than the Group 1 elements. Their 1st ionization energy values are higher than those of the Group 1 ...
... They tend to form molecular compounds in which electrons are shared with other atoms. Reactivity of these elements tends to decrease as atomic number increases. These elements tend to be more reactive than the Group 1 elements. Their 1st ionization energy values are higher than those of the Group 1 ...
PHY4605–Introduction to Quantum Mechanics II Spring 1997 Problem Set 4 Jan. 31, 2005
... ρ(r) = 4πr0 = 0 r > r0 (a) To see how this distributed charge affects the ground state energy, use classical physics (Gauss’s law) to find the classical potential V1 (r) associated with the charge density above, and define the perturbation to the point-proton model to be δV (r) = V1 (r) − V0 (r). Us ...
... ρ(r) = 4πr0 = 0 r > r0 (a) To see how this distributed charge affects the ground state energy, use classical physics (Gauss’s law) to find the classical potential V1 (r) associated with the charge density above, and define the perturbation to the point-proton model to be δV (r) = V1 (r) − V0 (r). Us ...
General CHemistry Unit 2 Homework Notes
... Solids have a fixed shape. In a solid the particles are closely packed together. Each particle in a solid is held in one position and vibrates around that position. The particles in a liquid stay relatively close together, but they can move around each other. Gas particles are far apart; they move r ...
... Solids have a fixed shape. In a solid the particles are closely packed together. Each particle in a solid is held in one position and vibrates around that position. The particles in a liquid stay relatively close together, but they can move around each other. Gas particles are far apart; they move r ...
Review 2 key - Home [www.petoskeyschools.org]
... 21 Draw a picture of Phosphorous, including p+, n0, and e- in the correct energy levels (be able to do this for all elements 1-20 on the periodic table) 17 p+ 17 n0 ...
... 21 Draw a picture of Phosphorous, including p+, n0, and e- in the correct energy levels (be able to do this for all elements 1-20 on the periodic table) 17 p+ 17 n0 ...
History of "s,p,d,f"
... The concept of spectral “terms” and the use of series names such as principal, sharp, etc., has now passed from common use, replaced by the quantitative understanding of atomic structure provided by quantum mechanics. However, the notational shorthand used by the early spectroscopists was adapted an ...
... The concept of spectral “terms” and the use of series names such as principal, sharp, etc., has now passed from common use, replaced by the quantitative understanding of atomic structure provided by quantum mechanics. However, the notational shorthand used by the early spectroscopists was adapted an ...
Atomic Structure, Eelectronic Bonding, Periodicity, orbitals
... – Theoretically, this series continues on to g,h,i, etc. orbitals. • Practically speaking atoms that have been discovered or made up to this point in time only have electrons in s, p, d, or f orbitals in their ground state configurations. • Each wave function with an allowed combination of n, l, and ...
... – Theoretically, this series continues on to g,h,i, etc. orbitals. • Practically speaking atoms that have been discovered or made up to this point in time only have electrons in s, p, d, or f orbitals in their ground state configurations. • Each wave function with an allowed combination of n, l, and ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.




















![Review 2 key - Home [www.petoskeyschools.org]](http://s1.studyres.com/store/data/000860497_1-e3bea510ba504d09bc42d6f5e4936390-300x300.png)


