FE Review Chemistry - UTSA College of Engineering
... the nucleus • Element: a substance only composed of one type of atom • Isotope: element with the same number of protons but different atomic masses ...
... the nucleus • Element: a substance only composed of one type of atom • Isotope: element with the same number of protons but different atomic masses ...
Document
... the radial direction to become infinite. But in the Bohr atom the electron does not have such radial motion caused by this uncertainty effect. So in this ...
... the radial direction to become infinite. But in the Bohr atom the electron does not have such radial motion caused by this uncertainty effect. So in this ...
Chemistry Unit Test Study Guide (2012-2013)
... Shown below is the Bohr model of the atom, proposed by scientist Niels Bohr. Scientists create models to describe atoms, because __________________________________. Subatomic Mass Location in the Charge Particle (in amu) atom Proton Electron Neutron Nucleus The atom is made up of mostly ___________ ...
... Shown below is the Bohr model of the atom, proposed by scientist Niels Bohr. Scientists create models to describe atoms, because __________________________________. Subatomic Mass Location in the Charge Particle (in amu) atom Proton Electron Neutron Nucleus The atom is made up of mostly ___________ ...
chapter 2 - Scranton Prep Biology
... in the object's position. Weight is the measureof how strongly an object is pulled by earth's gravity, and it varies with distance from the earth's center. Thi key point is that the mass of a body does not vary with its position, whereasweight does. So, for all practical purposes-as long as we are e ...
... in the object's position. Weight is the measureof how strongly an object is pulled by earth's gravity, and it varies with distance from the earth's center. Thi key point is that the mass of a body does not vary with its position, whereasweight does. So, for all practical purposes-as long as we are e ...
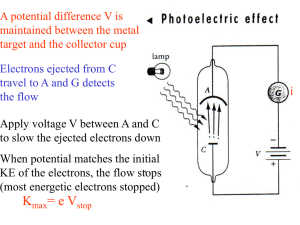
V stop f
... E=hf =>an electron absorbs one photon and gains energy hf (this process is independent of the intensity) • not expected classically! => increase intensity or wait longer for electron to absorb enough energy ...
... E=hf =>an electron absorbs one photon and gains energy hf (this process is independent of the intensity) • not expected classically! => increase intensity or wait longer for electron to absorb enough energy ...
46 Pd Palladium 106.4
... Name_______________________________________________ Date__________________ Period____ Ch4 Pre-assessment ...
... Name_______________________________________________ Date__________________ Period____ Ch4 Pre-assessment ...
Topological Insulators
... entangling many sorts of entities, typically identical atom or photon systems. But it has never been accomplished between an atomic system and a solid-state system such as a quantum dot in a semiconductor microcavity. Now two researchers have devised an experiment in which the quantum state of a sin ...
... entangling many sorts of entities, typically identical atom or photon systems. But it has never been accomplished between an atomic system and a solid-state system such as a quantum dot in a semiconductor microcavity. Now two researchers have devised an experiment in which the quantum state of a sin ...
Bonding in Atoms
... • Attraction of free floating electrons in the cloud • Flow of electrons allows for great conductivity • Also allows for malleability • Often arranged in a crystalline structure • Alloys is a mixture that includes at least one metal and enhances the properties of the metal ...
... • Attraction of free floating electrons in the cloud • Flow of electrons allows for great conductivity • Also allows for malleability • Often arranged in a crystalline structure • Alloys is a mixture that includes at least one metal and enhances the properties of the metal ...
Small Business Success on the Web
... Two atoms can share more than one pair of electrons double bonds (2 pairs of electrons) triple bonds (3 pairs of electrons) ...
... Two atoms can share more than one pair of electrons double bonds (2 pairs of electrons) triple bonds (3 pairs of electrons) ...
Ψ (x,t) = | Ψ (x,t) - University of Notre Dame
... Let’s assume V(x) = 0 between x=0 and x=L, and is infinite everywhere else…. Then, independent of energy, the electron cannot get out. Classically just bounces to and fro at constant speed, and the probability of being any one position inside (along x) is the same. Quantum mechanics: solve Schroding ...
... Let’s assume V(x) = 0 between x=0 and x=L, and is infinite everywhere else…. Then, independent of energy, the electron cannot get out. Classically just bounces to and fro at constant speed, and the probability of being any one position inside (along x) is the same. Quantum mechanics: solve Schroding ...
Online Course Evaluation Chapters 15-20
... (a) are the same for all elements (b) are characteristic of the particular element (c) are evenly distributed throughout the entire visible spectrum (d) are different from the wavelength in its darkline spectrum ...
... (a) are the same for all elements (b) are characteristic of the particular element (c) are evenly distributed throughout the entire visible spectrum (d) are different from the wavelength in its darkline spectrum ...
If electrons did not obey the Pauli exclusion Principle then….
... The electrons in an atom would annihilate with the protons in the nucleus The electrons in an atom would all have the same energy The electrons would repel each other preventing the formation of atoms The electrons in an atom would have a continuous range of energies rather than lying in discrete le ...
... The electrons in an atom would annihilate with the protons in the nucleus The electrons in an atom would all have the same energy The electrons would repel each other preventing the formation of atoms The electrons in an atom would have a continuous range of energies rather than lying in discrete le ...
Solution #3 - Temple University Department of Physics
... total angular momentum of the atom is F = J + I, where I is the nuclear spin. The eigenvalues of J 2 and F 2 are J(J + 1)~2 and F (F + 1)~2 respectively. a. What are the possible values of the quantum number J and F for a deuterium atom in the 1s ground state? In the 1s ground state of the deuterium ...
... total angular momentum of the atom is F = J + I, where I is the nuclear spin. The eigenvalues of J 2 and F 2 are J(J + 1)~2 and F (F + 1)~2 respectively. a. What are the possible values of the quantum number J and F for a deuterium atom in the 1s ground state? In the 1s ground state of the deuterium ...
chm 1045
... EXERCISE 7.3 : The following are representative wavelengths in the infrared, ultraviolet and x-ray regions of the electromagnetic spectrum, respectively: 1.0 x 10-6 m, 1.0 x 10-8 m and 1.0 x 10-10 m. • What is the energy of a photon of each radiation? • Which has the greatest amount of energy per ph ...
... EXERCISE 7.3 : The following are representative wavelengths in the infrared, ultraviolet and x-ray regions of the electromagnetic spectrum, respectively: 1.0 x 10-6 m, 1.0 x 10-8 m and 1.0 x 10-10 m. • What is the energy of a photon of each radiation? • Which has the greatest amount of energy per ph ...
Lecture 1 - UW Canvas
... a cathode-ray tube in 1897. By applying electric and magnetic fields to the ray and observing that it deflects, he concluded that the ray is negatively charged. By measuring the amount of deflection, he measured the charge-to-mass ratio. The particles in the ray always had the same ratio, so he conc ...
... a cathode-ray tube in 1897. By applying electric and magnetic fields to the ray and observing that it deflects, he concluded that the ray is negatively charged. By measuring the amount of deflection, he measured the charge-to-mass ratio. The particles in the ray always had the same ratio, so he conc ...
Chemistry 3211 – Coordination Chemistry Part 4 Electronic Spectra
... way of writing electron configurations that allows us to not only describe the ground state, but any possible excited states as well. We can do this by describing the electronic state according to its orbital and spin degeneracy. For two electrons in a p orbital, we can say that electron 1 will have ...
... way of writing electron configurations that allows us to not only describe the ground state, but any possible excited states as well. We can do this by describing the electronic state according to its orbital and spin degeneracy. For two electrons in a p orbital, we can say that electron 1 will have ...
Units 1-6
... I can describe ways to separate heterogeneous mixtures (filtration, settling), and ways to separate homogeneous mixtures (distillation, chromatography, crystallization) I know that separation of compounds requires chemical reactions such as electrolysis, and that elements can’t be separated because ...
... I can describe ways to separate heterogeneous mixtures (filtration, settling), and ways to separate homogeneous mixtures (distillation, chromatography, crystallization) I know that separation of compounds requires chemical reactions such as electrolysis, and that elements can’t be separated because ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.