Chapter 9 The Atom - Bakersfield College
... The quantity that varies in a matter wave is called the wave function (y). The square of the wave function (y2) is called the probability density. For a given object, the greater the probability density at a certain time and place, the greater the likelihood of finding the object there at that time. ...
... The quantity that varies in a matter wave is called the wave function (y). The square of the wave function (y2) is called the probability density. For a given object, the greater the probability density at a certain time and place, the greater the likelihood of finding the object there at that time. ...
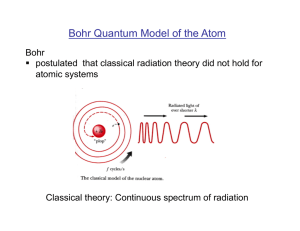
Bohr Quantum Model of the Atom
... § postulated that the electron orbital momentum is quantized Justification of Bohr’s postulates: comparison with experimental observations! ...
... § postulated that the electron orbital momentum is quantized Justification of Bohr’s postulates: comparison with experimental observations! ...
$doc.title
... statement, and assuming classical particles, a calculation of the molar specific heat at constant volume of such a metal would be the following: Cv = 3R + 3R/2 = 9R/2, ...
... statement, and assuming classical particles, a calculation of the molar specific heat at constant volume of such a metal would be the following: Cv = 3R + 3R/2 = 9R/2, ...
Section 2 The Structure of the Atom Discovery of the Electron
... • An atom is the smallest particle of an element that retains the chemical properties of that element. • The nucleus is a very small region located at the center of an atom. • The nucleus is made up of at least one positively charged particle called a proton and usually one or more neutral particles ...
... • An atom is the smallest particle of an element that retains the chemical properties of that element. • The nucleus is a very small region located at the center of an atom. • The nucleus is made up of at least one positively charged particle called a proton and usually one or more neutral particles ...
Focusing light with the power of 1000 Hoover Dams
... high-energy in laser wake fields just as dolphins gain energy by swimming in phase with the water wave in the wake of a ship. Such a laser wake-field accelerator was first proposed in 1979 by Toshiki Tajima and John M. Dawson, both then at the University of California at Los Angeles. The process of ...
... high-energy in laser wake fields just as dolphins gain energy by swimming in phase with the water wave in the wake of a ship. Such a laser wake-field accelerator was first proposed in 1979 by Toshiki Tajima and John M. Dawson, both then at the University of California at Los Angeles. The process of ...
Fermium does not occur naturally in the Earth`s crust. It was first
... neutron – an elementary particle with no net charge and a rest mass of about 1.675 × 10–27 kg, slightly more than that of the proton. All atoms contain neutrons in their nucleus except for protium (1H). [return] proton – an elementary particle having a rest mass of about 1.673 × 10–27 kg, slightly l ...
... neutron – an elementary particle with no net charge and a rest mass of about 1.675 × 10–27 kg, slightly more than that of the proton. All atoms contain neutrons in their nucleus except for protium (1H). [return] proton – an elementary particle having a rest mass of about 1.673 × 10–27 kg, slightly l ...
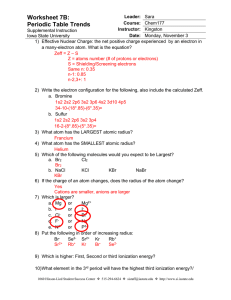
worksheet 7b answers - Iowa State University
... Iowa State University 1) Effective Nuclear Charge: the net positive charge experienced by an electron in a many-electron atom. What is the equation? Zeff = Z – S Z = atoms number (# of protons or electrons) S = Shielding/Screening electrons Same n: 0.35 n-1: 0.85 n-2,3+: 1 ...
... Iowa State University 1) Effective Nuclear Charge: the net positive charge experienced by an electron in a many-electron atom. What is the equation? Zeff = Z – S Z = atoms number (# of protons or electrons) S = Shielding/Screening electrons Same n: 0.35 n-1: 0.85 n-2,3+: 1 ...
Teaching the Standard Model in IB Physics by Debra Blake
... Physicist call the framework that describes the interaction between elementary building blocks (quarks and leptons) and the force carriers (bosons) the Standard Model. Gravity is not yet part of this framework. The biggest success of the Standard Model is the unification of the electromagnetic and t ...
... Physicist call the framework that describes the interaction between elementary building blocks (quarks and leptons) and the force carriers (bosons) the Standard Model. Gravity is not yet part of this framework. The biggest success of the Standard Model is the unification of the electromagnetic and t ...
In Search of Giants Worksheet
... a. What particle carries or accounts for the strong nuclear force? ...
... a. What particle carries or accounts for the strong nuclear force? ...
(2)
... found that the explanation of the black body radiation is impossible unless light behaves as discrete energy quantum, known as photons [1, 2].This means that light has dual wave and particle nature .The particle behaviour of light waves motivates De Broglie to suggest that particles like electrons c ...
... found that the explanation of the black body radiation is impossible unless light behaves as discrete energy quantum, known as photons [1, 2].This means that light has dual wave and particle nature .The particle behaviour of light waves motivates De Broglie to suggest that particles like electrons c ...
Lecture 15
... into the same state. So this particle goes into the same orbital state ψ100(r), but has opposite spin. The total energy is about – 2× 54.4 eV = - 108.8 eV. I say about, because there is also the interaction energy of the two electrons, which comes about because of their mutual repulsion. It is a lar ...
... into the same state. So this particle goes into the same orbital state ψ100(r), but has opposite spin. The total energy is about – 2× 54.4 eV = - 108.8 eV. I say about, because there is also the interaction energy of the two electrons, which comes about because of their mutual repulsion. It is a lar ...
Schr dinger Equation
... Let us begin by stating that there are certain things which we must simply accept and there is no way to prove them. They are the things that we postulate must be true. They cannot be proven but if they are accepted then what follows bears out in the real world. As such QM offers a tool to predict t ...
... Let us begin by stating that there are certain things which we must simply accept and there is no way to prove them. They are the things that we postulate must be true. They cannot be proven but if they are accepted then what follows bears out in the real world. As such QM offers a tool to predict t ...
If electrons did not obey the Pauli exclusion Principle then….
... Hence, there are...... More photons in one joule of red light than in one joule of blue More photons in one joule of blue light than in one joule of red The same number of photons in one joule of red light as in one joule of blue ...
... Hence, there are...... More photons in one joule of red light than in one joule of blue More photons in one joule of blue light than in one joule of red The same number of photons in one joule of red light as in one joule of blue ...
Chemistry Week 04 - nchsdduncanchem1
... Electrons in the same orbital must have opposite spins. ...
... Electrons in the same orbital must have opposite spins. ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.