Wave Particle Duality Power Point NOTES
... De Broglie’s Equation • Broglie’s stated that an object in motion behaves as both particles & waves, just as light does. • Electrons are WAVES in FIXED LOCATIONS which means they are QUANTIZED (quantity) wavelengths. ...
... De Broglie’s Equation • Broglie’s stated that an object in motion behaves as both particles & waves, just as light does. • Electrons are WAVES in FIXED LOCATIONS which means they are QUANTIZED (quantity) wavelengths. ...
Structure of matter.
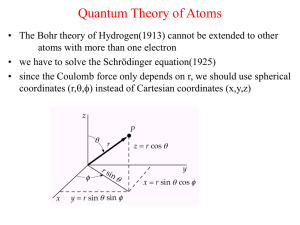
... The solution of the Schrödinger equation for the electron in the hydrogen atom leads to the values of the energies of the orbital electron. The solution of the Schrödinger equation often leads to numerical coefficients which determine the possible values of energy. These numerical coefficients a ...
... The solution of the Schrödinger equation for the electron in the hydrogen atom leads to the values of the energies of the orbital electron. The solution of the Schrödinger equation often leads to numerical coefficients which determine the possible values of energy. These numerical coefficients a ...
Chapter 7 (Lecture 10) Hydrogen Atom The explanation of
... two parts. The first part is called the asymptotic behavior, referring to the solution at very large distance from the proton or very close to the proton. The asymptotic behaviour of the equation ...
... two parts. The first part is called the asymptotic behavior, referring to the solution at very large distance from the proton or very close to the proton. The asymptotic behaviour of the equation ...
1_Quantum theory_ introduction and principles
... 1.2 The Schrödinger Equation From the wave-particle duality, the concepts of classical physics (CP) have to be abandoned to describe microscopic systems. The dynamics of microscopic systems will be described in a new theory: the quantum theory (QT). A wave, called wavefunction (r,t), is associat ...
... 1.2 The Schrödinger Equation From the wave-particle duality, the concepts of classical physics (CP) have to be abandoned to describe microscopic systems. The dynamics of microscopic systems will be described in a new theory: the quantum theory (QT). A wave, called wavefunction (r,t), is associat ...
Eighth International Conference on Geometry, Integrability and Quantization
... was that elementary particles need not be pointlike. Being extended and non rigid is a better conception. Rather than conceiving the particle as a bulk of fluid, we have supposed that it is composed of pointlike quantum modes. This enabled the construction of our Geometro-Differential Model (G-D-M) ...
... was that elementary particles need not be pointlike. Being extended and non rigid is a better conception. Rather than conceiving the particle as a bulk of fluid, we have supposed that it is composed of pointlike quantum modes. This enabled the construction of our Geometro-Differential Model (G-D-M) ...
Physics 880.06: Problem Set 5
... 1. Consider the Ginzburg-Landau differential equation for ψ as applied to an order parameter ψ which varies in only one spatial direction, say z. If there is no vector potential, this differential equation can be written h̄2 ′′ ψ (z) = 0, αψ + β|ψ| ψ − 2m∗ ...
... 1. Consider the Ginzburg-Landau differential equation for ψ as applied to an order parameter ψ which varies in only one spatial direction, say z. If there is no vector potential, this differential equation can be written h̄2 ′′ ψ (z) = 0, αψ + β|ψ| ψ − 2m∗ ...
Charged Particles in Magnetic Fields
... Suppose a particle with charge q and mass m moves with velocity vector v. If a force F acts in the same direction as the velocity v then the particle continues to move in the same direction, but it speeds up. This is what an electric field can do to charged particles. We can describe it a bit differ ...
... Suppose a particle with charge q and mass m moves with velocity vector v. If a force F acts in the same direction as the velocity v then the particle continues to move in the same direction, but it speeds up. This is what an electric field can do to charged particles. We can describe it a bit differ ...
슬라이드 1
... The Schrödinger Equation Hamiltonian operator energy & wavefunction (solving a partial differential equation) with ...
... The Schrödinger Equation Hamiltonian operator energy & wavefunction (solving a partial differential equation) with ...
0117 Lecture Notes - AP Physics 1 Equations to
... Flipping Physics Lecture Notes: AP Physics 1: Equations to Memorize ...
... Flipping Physics Lecture Notes: AP Physics 1: Equations to Memorize ...
PHYSICS 215 - Thermodynamics and Modern Physics Name:
... What is the minimum angle between L and the z axis? ...
... What is the minimum angle between L and the z axis? ...
Quantum Theory 1 - Class Exercise 4
... Quantum Theory 1 - Class Exercise 4 1. Consider a Hamiltonian which describes a one dimensional system of two particles of masses m1 and m2 moving in a potential that depends only on the distance between them. Ĥ = ...
... Quantum Theory 1 - Class Exercise 4 1. Consider a Hamiltonian which describes a one dimensional system of two particles of masses m1 and m2 moving in a potential that depends only on the distance between them. Ĥ = ...
File
... carbon-14 and providing the impetus for modern carbon dating. As part of this research, he discovered the proton and neutron, the latter in cooperation with James Chadwick. He is also the only native New Zealander with an element named after him (Rutherfordium, atomic number 104). 10. Erwin Schrödin ...
... carbon-14 and providing the impetus for modern carbon dating. As part of this research, he discovered the proton and neutron, the latter in cooperation with James Chadwick. He is also the only native New Zealander with an element named after him (Rutherfordium, atomic number 104). 10. Erwin Schrödin ...
Modern Physics
... powerful techniques for solving problems in quantum physics In general the equation is applied in three dimensions of space as well as time For simplicity we will consider only the one dimensional, time independent case The wave equation for a wave of displacement y and velocity v is given by ...
... powerful techniques for solving problems in quantum physics In general the equation is applied in three dimensions of space as well as time For simplicity we will consider only the one dimensional, time independent case The wave equation for a wave of displacement y and velocity v is given by ...
2.1.7 particle movement in magnetic fields
... can be to change speed or direction. The effect of a magnetic field on a charged particle can only be to change its direction. This is because the force applied is always perpendicular to its motion. ...
... can be to change speed or direction. The effect of a magnetic field on a charged particle can only be to change its direction. This is because the force applied is always perpendicular to its motion. ...
INFORMATION ON MASTER`S THESIS 1. Full name: VU THI MINH
... 11. Summary of the finding of the thesis: Thesis: “Anomalous magnetic moment of electrons and methods Pauli – Villars in quantum field theory” study the anomalous magnetic moment of the electron in quantum field theory. The main complementary magnetic moment based on perturbation theory via covarian ...
... 11. Summary of the finding of the thesis: Thesis: “Anomalous magnetic moment of electrons and methods Pauli – Villars in quantum field theory” study the anomalous magnetic moment of the electron in quantum field theory. The main complementary magnetic moment based on perturbation theory via covarian ...