Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... The theory was found to be extremely successful in describing nature (see rest of the course), but as two of its fathers put it: “To try and stop all attempts to pass beyond the present viewpoint of quantum physics could be very dangerous for the progress of science and would furthermore be contrary ...
... The theory was found to be extremely successful in describing nature (see rest of the course), but as two of its fathers put it: “To try and stop all attempts to pass beyond the present viewpoint of quantum physics could be very dangerous for the progress of science and would furthermore be contrary ...
CHAPTER 7: The Hydrogen Atom
... The atom of modern physics can be symbolized only through a partial differential equation in an abstract space of many dimensions. All its qualities are inferential; no material properties can be directly attributed to it. An understanding of the atomic world in that primary sensuous fashion…is impo ...
... The atom of modern physics can be symbolized only through a partial differential equation in an abstract space of many dimensions. All its qualities are inferential; no material properties can be directly attributed to it. An understanding of the atomic world in that primary sensuous fashion…is impo ...
Lecture 18: Intro. to Quantum Mechanics
... • Louis de Broglie suggests that for the e- orbits envisioned by Bohr, only certain orbits are allowed since they satisfy the standing wave condition. ...
... • Louis de Broglie suggests that for the e- orbits envisioned by Bohr, only certain orbits are allowed since they satisfy the standing wave condition. ...
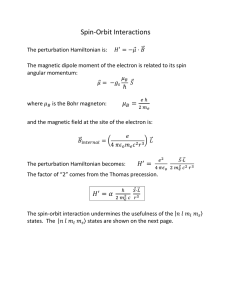
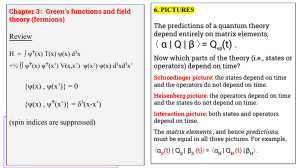
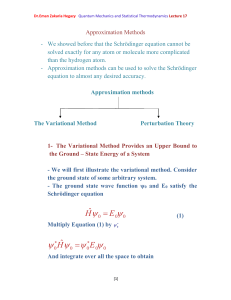
α | Q | β 〉= Q (t) . 〈 Review
... The initial and final states , i.e., as t ⟶ −∞ and +∞, are free particles, i.e., eigenstates of H0. The state experiences the interactions H1 during the time -1/ε ≲ t ≲ +1/ε . ...
... The initial and final states , i.e., as t ⟶ −∞ and +∞, are free particles, i.e., eigenstates of H0. The state experiences the interactions H1 during the time -1/ε ≲ t ≲ +1/ε . ...
Thesis Presentation Mr. Joshuah T. Heath Department of Physics
... analytical expression can be derived for the partition function at any density and chemical potential. In the canonical ensemble, the total number of particles, N, is fixed and an expression for the partition function can only be generated via a complicated recursion relation. In this work we apply ...
... analytical expression can be derived for the partition function at any density and chemical potential. In the canonical ensemble, the total number of particles, N, is fixed and an expression for the partition function can only be generated via a complicated recursion relation. In this work we apply ...
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical
... We now minimize E () with respect to by differentiating with respect to and setting the result equal to zero. We solve the equation: ...
... We now minimize E () with respect to by differentiating with respect to and setting the result equal to zero. We solve the equation: ...
Particle Physics
... from mutually independent causes (additivity of the two terms)” — Einstein (1909) Attempts at obtaining this from dynamics (as time averages) could only give one or the other term… ...
... from mutually independent causes (additivity of the two terms)” — Einstein (1909) Attempts at obtaining this from dynamics (as time averages) could only give one or the other term… ...
Infra-red Quantum Effects in de Sitter Space
... Trace of energy-momentum tensor We may simply evaluate the trace of the energy-momentum tensor ...
... Trace of energy-momentum tensor We may simply evaluate the trace of the energy-momentum tensor ...
Midterm Solution
... 1b. Does the improbability she/he mentions mean that there is still a finite probability that a quantum mechanical object could be in a place where its total energy is less than its potential energy? Yes P in principle No (no is acceptable if well argued due to the measurement problem, it’s no in pr ...
... 1b. Does the improbability she/he mentions mean that there is still a finite probability that a quantum mechanical object could be in a place where its total energy is less than its potential energy? Yes P in principle No (no is acceptable if well argued due to the measurement problem, it’s no in pr ...