Chap 6.
... values, ± 12 . The electron is said to be an elementary particle of spin 12 . The proton and neutron also have spin 12 and belong to the classification of particles called fermions, which are govened by the Pauli exclusion principle. Other particles, including the photon, have integer values of spi ...
... values, ± 12 . The electron is said to be an elementary particle of spin 12 . The proton and neutron also have spin 12 and belong to the classification of particles called fermions, which are govened by the Pauli exclusion principle. Other particles, including the photon, have integer values of spi ...
doc - Seth Baum
... a. What is heat 6) Equation describing the gravitational potential energy between two objects d. What is Ug = - G m1 m2 / r 7) The SI unit that the spring constant is measured in e. What is Newtons per Meter or Kilograms per Second Squared 8) The SI unit that acceleration is measured in f. What is M ...
... a. What is heat 6) Equation describing the gravitational potential energy between two objects d. What is Ug = - G m1 m2 / r 7) The SI unit that the spring constant is measured in e. What is Newtons per Meter or Kilograms per Second Squared 8) The SI unit that acceleration is measured in f. What is M ...
0_2_SA_LarmorPrecession
... knowing the relevant constants from available data tables, it should be verified that the following equation closely approximates the resonance frequency-field criterion for ESR. 1 Gauss = 2.8 MHz for a free electron spin with g=2 ...
... knowing the relevant constants from available data tables, it should be verified that the following equation closely approximates the resonance frequency-field criterion for ESR. 1 Gauss = 2.8 MHz for a free electron spin with g=2 ...
File - SPHS Devil Physics
... a. Observations: Much of the work towards a quantum theory of atoms was guided by the need to explain the observed patterns in atomic spectra. The first quantum model of matter is the Bohr model for hydrogen. (1.8) b. Paradigm shift: The acceptance of the wave–particle duality paradox for light and ...
... a. Observations: Much of the work towards a quantum theory of atoms was guided by the need to explain the observed patterns in atomic spectra. The first quantum model of matter is the Bohr model for hydrogen. (1.8) b. Paradigm shift: The acceptance of the wave–particle duality paradox for light and ...
semester ii
... observables in Schrodinger picture and Heisenberg picture - Heisenberg equation of motion Ehrenfest’s theorem - time evolution of base kets - transition amplitude – energy eigenket and eigen values of a simple harmonic oscillator using creation and annihilation operators Identical particles Identity ...
... observables in Schrodinger picture and Heisenberg picture - Heisenberg equation of motion Ehrenfest’s theorem - time evolution of base kets - transition amplitude – energy eigenket and eigen values of a simple harmonic oscillator using creation and annihilation operators Identical particles Identity ...
Using Pink Diamond to Detect Small Magnetic Fields and Break
... diamond as a qubit and evaluate its performance in a quantum computer. EPR is a technique used to study samples that have unpaired spins. Spin is a property of quantum particles and there is no classical equivalence of this property. A particle can have fractional spin (e.g. Spin=1/2 for an ele ...
... diamond as a qubit and evaluate its performance in a quantum computer. EPR is a technique used to study samples that have unpaired spins. Spin is a property of quantum particles and there is no classical equivalence of this property. A particle can have fractional spin (e.g. Spin=1/2 for an ele ...
l - Evergreen
... H-atom wavefunctions ↔ electron probability distributions: l = angular momentum wavenumber ...
... H-atom wavefunctions ↔ electron probability distributions: l = angular momentum wavenumber ...
Transparancies for Feynman Graphs
... This gives anti-screening effect. Coupling strength falls as |q2| increases ...
... This gives anti-screening effect. Coupling strength falls as |q2| increases ...
Quantum1
... events will behave in a statistically predictable way. probability for an electron to be found between x and x+dx ...
... events will behave in a statistically predictable way. probability for an electron to be found between x and x+dx ...
Electron transport in 3D topological insulators
... Topological insulators (TI) are new states of matter, where there exist topologically protected surface and edge states which exhibit spin‐momentum locking. Here, we investigate the theory of electron transport on the topological surface states of topological insulators. The techniq ...
... Topological insulators (TI) are new states of matter, where there exist topologically protected surface and edge states which exhibit spin‐momentum locking. Here, we investigate the theory of electron transport on the topological surface states of topological insulators. The techniq ...
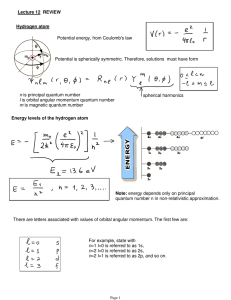
Lecture 12: Review.
... interactions of electrons with the quantized electromagnetic field. In QED, a quantized radiation field in the lowest-energy state of NOT the one with ZERO electromagnetic fields, but there exist zero-point oscillations. Then, there are non-zero electromagnetic fields that are present even in the ab ...
... interactions of electrons with the quantized electromagnetic field. In QED, a quantized radiation field in the lowest-energy state of NOT the one with ZERO electromagnetic fields, but there exist zero-point oscillations. Then, there are non-zero electromagnetic fields that are present even in the ab ...
50 POINTS - University at Albany
... (b.) Product of delta-x and delta-p must be greater than or equal to h-bar/2, where delta-p is equal to (1.15 * 6.626e-25) kg*m/s minus (6.626e-25 / 1.15) kg*m/s, using part (a.) (Also is acceptable if doing / and * 0.85. Very similar results). Δx <= h-bar / (2*Δp) = [h / (2 * pi)] / (2 * Δp) = 6.62 ...
... (b.) Product of delta-x and delta-p must be greater than or equal to h-bar/2, where delta-p is equal to (1.15 * 6.626e-25) kg*m/s minus (6.626e-25 / 1.15) kg*m/s, using part (a.) (Also is acceptable if doing / and * 0.85. Very similar results). Δx <= h-bar / (2*Δp) = [h / (2 * pi)] / (2 * Δp) = 6.62 ...








![ABSTRACT – Condensed Matter Physics [ORIGINAL]](http://s1.studyres.com/store/data/005325689_1-bd59cbe3830dc734895532d6f7679a5c-300x300.png)