Medical Biophysics
... Atoms cannot be subdivided, created, or destroyed. Atoms of different elements combine in simple whole-number ratios to form chemical compounds In chemical reactions, atoms are combined, separated, or rearranged. ...
... Atoms cannot be subdivided, created, or destroyed. Atoms of different elements combine in simple whole-number ratios to form chemical compounds In chemical reactions, atoms are combined, separated, or rearranged. ...
Chapter 30 Notes - Valdosta State University
... The Quantum Mechanical Picture of the Hydrogen Atom The Bohr model of the atom uses a single number to identify the state of an electron in the hydrogen atom. It is called a quantum number since it can only have certain discrete values such as 1, 2, 3, etc. Electrons with slightly different states ...
... The Quantum Mechanical Picture of the Hydrogen Atom The Bohr model of the atom uses a single number to identify the state of an electron in the hydrogen atom. It is called a quantum number since it can only have certain discrete values such as 1, 2, 3, etc. Electrons with slightly different states ...
Posttest for Uncertainty Principle Part 1
... observable A first and then measure observable B immediately afterwards; (II) directly measure B without measuring A first. The initial state when the first measurement is performed in each of these two situations is the same, i.e., a generic state , which is not an eigenstate of  or B̂ . Will t ...
... observable A first and then measure observable B immediately afterwards; (II) directly measure B without measuring A first. The initial state when the first measurement is performed in each of these two situations is the same, i.e., a generic state , which is not an eigenstate of  or B̂ . Will t ...
Monday, Mar. 23, 2015
... Importance of Bohr’s Model • Demonstrated the need for Plank’s constant in understanding the atomic structure • Assumption of quantized angular momentum which led to quantization of other quantities, r, v and E as ...
... Importance of Bohr’s Model • Demonstrated the need for Plank’s constant in understanding the atomic structure • Assumption of quantized angular momentum which led to quantization of other quantities, r, v and E as ...
Chapter 4 Section 2
... arranged in circular paths (orbits) around the nucleus Answered Rutherford’s ?—electrons in a particular path have a fixed energy, they do NOT lose energy and fall into the nucleus Energy level—region around nucleus where it is likely to be moving, similar to rungs on a ladder but not equally spaced ...
... arranged in circular paths (orbits) around the nucleus Answered Rutherford’s ?—electrons in a particular path have a fixed energy, they do NOT lose energy and fall into the nucleus Energy level—region around nucleus where it is likely to be moving, similar to rungs on a ladder but not equally spaced ...
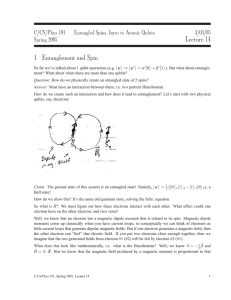
Lecture 14 1 Entanglement and Spin
... Electron spins are hardly the only quantum system that can be entangled, so we might want to talk about general entanglement through “interaction Hamiltonians.” In the most general sense, we can talk about two quantum numbers x1 and x2 (i.e. quantized physical observables) that yield the following H ...
... Electron spins are hardly the only quantum system that can be entangled, so we might want to talk about general entanglement through “interaction Hamiltonians.” In the most general sense, we can talk about two quantum numbers x1 and x2 (i.e. quantized physical observables) that yield the following H ...
General Chemistry I
... Dalton's Atomic Theory (1803) • Matter is composed of indivisible particles - atoms. • An element is composed of only one kind of atom. These atoms in a particular element have the same properties such as mass, size, or even shape. • A compound is composed of two or more elements combined in fixed r ...
... Dalton's Atomic Theory (1803) • Matter is composed of indivisible particles - atoms. • An element is composed of only one kind of atom. These atoms in a particular element have the same properties such as mass, size, or even shape. • A compound is composed of two or more elements combined in fixed r ...
Physical Chemistry Composite systems Adding angular momenta
... electrons are in the lowestΨspatial (r1 , r2 , r3 ) = Ψ1s (r1 )Ψ1s (r2 ) Ψ1s (r3 ) energy state Example: lithium atom Cannot happen Pauli’s principle: there can never be two equivalent electrons in an atom for which the values of all the quantum α⎞ ...
... electrons are in the lowestΨspatial (r1 , r2 , r3 ) = Ψ1s (r1 )Ψ1s (r2 ) Ψ1s (r3 ) energy state Example: lithium atom Cannot happen Pauli’s principle: there can never be two equivalent electrons in an atom for which the values of all the quantum α⎞ ...
Unit 3 Test - hrsbstaff.ednet.ns.ca
... ___ Combustibility is the ability of a substance to react with acids ___ Sugar disappearing in water is an example of a solution ___ Raisins in Raisin Bran are an example of a solution ___ Lighting a test tube of acetylene gas is an example of a reaction with acid ___ Lighting a test tube of acetyle ...
... ___ Combustibility is the ability of a substance to react with acids ___ Sugar disappearing in water is an example of a solution ___ Raisins in Raisin Bran are an example of a solution ___ Lighting a test tube of acetylene gas is an example of a reaction with acid ___ Lighting a test tube of acetyle ...
Standard EPS Shell Presentation
... appears as a line in a spectroscope. A spectroscope is a device that spreads light into its different colors. ...
... appears as a line in a spectroscope. A spectroscope is a device that spreads light into its different colors. ...
Quantum Number
... • De Broglie's hypothesis was soon confirmed in experiments that showed electron beams could be diffracted or bent as they passed through a slit much like light could. • The waves produced by an electron confined in its orbit about the nucleus sets up a standing wave of specific wavelength, energy a ...
... • De Broglie's hypothesis was soon confirmed in experiments that showed electron beams could be diffracted or bent as they passed through a slit much like light could. • The waves produced by an electron confined in its orbit about the nucleus sets up a standing wave of specific wavelength, energy a ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).