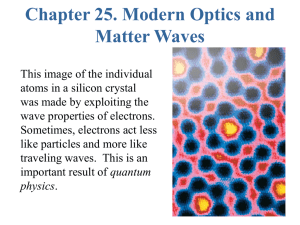
The buoyant force on an object totally submerged in a fluid depends
... come; for truth will stand, truth will endure." -- Joseph F. Smith ...
... come; for truth will stand, truth will endure." -- Joseph F. Smith ...
Chapt25_VGO
... • In 1927 Davisson and Germer were studying how electrons scatter from the surface of metals. • They found that electrons incident normal to the crystal face at a speed of 4.35 106 m/s scattered at ø = 50°. • This scattering can be interpreted as a mirror-like reflection from the atomic planes tha ...
... • In 1927 Davisson and Germer were studying how electrons scatter from the surface of metals. • They found that electrons incident normal to the crystal face at a speed of 4.35 106 m/s scattered at ø = 50°. • This scattering can be interpreted as a mirror-like reflection from the atomic planes tha ...
Physics 1C - University of California, San Diego
... This is known as emission spectra. Each line has a different wavelength (color). The elemental composition of the gas will tell what the resulting color lines will be. ...
... This is known as emission spectra. Each line has a different wavelength (color). The elemental composition of the gas will tell what the resulting color lines will be. ...
A critical analysis of the hydrino model
... and hence dubious (cf. e. g. [6]). This lack of theoretical consideration is particularly unfortunate in view of the wealth of experimental evidence that has been published in peer reviewed journals in favour of the hydrino model [1, 2, 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, ...
... and hence dubious (cf. e. g. [6]). This lack of theoretical consideration is particularly unfortunate in view of the wealth of experimental evidence that has been published in peer reviewed journals in favour of the hydrino model [1, 2, 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, ...
Fine and hyperfine structure of the hydrogen atom
... UNIT 4: Fine and hyperfine structure of the hydrogen atom measurements of the position of the electron can only be accurate up to ∼ λ0 . So, rather than being able to exactly determine the electron position, we can only determine a fuzzy ball about the size of λ0 . This is illustrated in Figure 1. ...
... UNIT 4: Fine and hyperfine structure of the hydrogen atom measurements of the position of the electron can only be accurate up to ∼ λ0 . So, rather than being able to exactly determine the electron position, we can only determine a fuzzy ball about the size of λ0 . This is illustrated in Figure 1. ...
hc1(4)notes
... The Uncertainty Principle • German physicist Werner Heisenberg proposed that any attempt to locate a specific electron with a photon knocks the electron off its course. ...
... The Uncertainty Principle • German physicist Werner Heisenberg proposed that any attempt to locate a specific electron with a photon knocks the electron off its course. ...
Atomic 1
... •We know that when the electron revolves around the nucleus gives rise to current loop and a magnetic field is associated with it. •Hence atomic electron possessing an angular momentum interacts with this magnetic field. ...
... •We know that when the electron revolves around the nucleus gives rise to current loop and a magnetic field is associated with it. •Hence atomic electron possessing an angular momentum interacts with this magnetic field. ...
QUANTUM MECHANICAL MODEL OF THE ATOM
... the study of the motions of the microscopic objects that have both observable wave like and particle like properties. • Quantum mechanics is based on a fundamental equation which is called Schrodinger equation. • Schrodinger’s equation: For a system (such as an atom or a molecule whose energy does n ...
... the study of the motions of the microscopic objects that have both observable wave like and particle like properties. • Quantum mechanics is based on a fundamental equation which is called Schrodinger equation. • Schrodinger’s equation: For a system (such as an atom or a molecule whose energy does n ...
Physics 411: Introduction to Quantum Mechanics
... Brief course description: Introduction to Quantum Mechanics is a two-semester course (411 and 412) and is mandatory for all physics majors pursuing the Academic Physics Concentration. Roughly speaking, 411 deals with the foundations and development of quantum theory whereas 412 is more geared toward ...
... Brief course description: Introduction to Quantum Mechanics is a two-semester course (411 and 412) and is mandatory for all physics majors pursuing the Academic Physics Concentration. Roughly speaking, 411 deals with the foundations and development of quantum theory whereas 412 is more geared toward ...
Prof. Bertrand Reulet, Université de Sherbrooke, Canada Talk: 23. May 2014
... Abstract: > Electrons in conductors have a disordered motion which cause random > fluctuations of the electrical current, a phenomenon commonly referred > to as "noise". In classical physics, the variance of these > fluctuations is simply proportional to the temperature. In a tiny > device placed at ...
... Abstract: > Electrons in conductors have a disordered motion which cause random > fluctuations of the electrical current, a phenomenon commonly referred > to as "noise". In classical physics, the variance of these > fluctuations is simply proportional to the temperature. In a tiny > device placed at ...
The Modern Atomic Model
... Bohr Model of the Atom (review) •Energy levels contain electrons. •Electrons travel around the nucleus. •Different orbitals varied by different quantum (energy). •Gaps between energy levels were not equal. ...
... Bohr Model of the Atom (review) •Energy levels contain electrons. •Electrons travel around the nucleus. •Different orbitals varied by different quantum (energy). •Gaps between energy levels were not equal. ...
Quantum Physics Cumulative Review
... 4. An electron orbits the nucleus in a Bohr hydrogen atom. Calculate radius, speed and energy for each of the following orbits: a) n = 4 b) n = 8 5. Based on your previous results, what wavelength photon is produced when the electron in a hydrogen atom drops from the 8th energy level to the 4th ene ...
... 4. An electron orbits the nucleus in a Bohr hydrogen atom. Calculate radius, speed and energy for each of the following orbits: a) n = 4 b) n = 8 5. Based on your previous results, what wavelength photon is produced when the electron in a hydrogen atom drops from the 8th energy level to the 4th ene ...
Document
... where the g-factor for electron gs is needed to obtain agreement with experimental observations. The g-factor for electron does not have classical analog. In 1928 Dirac developed a relativistic generalization of Schrödinger equation for electrons, which gave gs = 2, exactly. The observed value, howe ...
... where the g-factor for electron gs is needed to obtain agreement with experimental observations. The g-factor for electron does not have classical analog. In 1928 Dirac developed a relativistic generalization of Schrödinger equation for electrons, which gave gs = 2, exactly. The observed value, howe ...
Ch. 4-2 PowerPoint
... Orbital – a 3-dimensional region around the nucleus that indicates the probable location of an electron. ...
... Orbital – a 3-dimensional region around the nucleus that indicates the probable location of an electron. ...
Electromagnetic Radiation and Atomic Physics
... The “cloud” of electrons, on the other hand, occupies a volume with a diameter of about 0.1 nm (1 nm = 10-9 m). Since mp = 1836 me and mn = 1839 me, most of the mass of an atom is concentrated in its nucleus. An ion is an atom that has either a deficit or a surplus of electrons. The process of remov ...
... The “cloud” of electrons, on the other hand, occupies a volume with a diameter of about 0.1 nm (1 nm = 10-9 m). Since mp = 1836 me and mn = 1839 me, most of the mass of an atom is concentrated in its nucleus. An ion is an atom that has either a deficit or a surplus of electrons. The process of remov ...
IB Atomic and radioactivity Math questions
... This question is about the nuclear structure of the atom and atomic energy levels. When the electron was first discovered it led to the idea that an atom consists of a lump of positive charge in which the electrons are embedded. In 1912 Geiger and Marsden carried out an experiment to test the validi ...
... This question is about the nuclear structure of the atom and atomic energy levels. When the electron was first discovered it led to the idea that an atom consists of a lump of positive charge in which the electrons are embedded. In 1912 Geiger and Marsden carried out an experiment to test the validi ...
CHEM 121
... 16. The energy levels for the electron in a hydrogen atom are given by E = -Rhcn-2. Make a diagram using -1/n2 in place of the actual energies. Draw energy levels for n=1,2,3,4,5,6,7,∞; show the transitions for problem 15. 17. MULTIPLE CHOICE. Which equation gives the Bohr energy of the electron in ...
... 16. The energy levels for the electron in a hydrogen atom are given by E = -Rhcn-2. Make a diagram using -1/n2 in place of the actual energies. Draw energy levels for n=1,2,3,4,5,6,7,∞; show the transitions for problem 15. 17. MULTIPLE CHOICE. Which equation gives the Bohr energy of the electron in ...
ki̇mya
... • the electrons in an atom move at a certain distance from nucleus and their motions are stable . Each stationery state has a definite energy. • Electrons move in each stationary energy state in a circular orbital. These circular orbitals are called energy levels or shells. The possible states for t ...
... • the electrons in an atom move at a certain distance from nucleus and their motions are stable . Each stationery state has a definite energy. • Electrons move in each stationary energy state in a circular orbital. These circular orbitals are called energy levels or shells. The possible states for t ...
Probing the Orbital Energy of an Electron in an Atom
... precisely known, the uncertainty in momentum and therefore kinetic energy must be ...
... precisely known, the uncertainty in momentum and therefore kinetic energy must be ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).