What is Light?
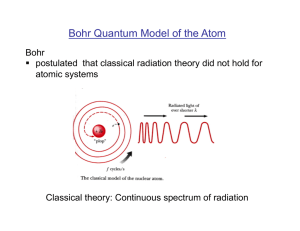
... Bohr Atomic Model (1912) • Combined planetary model and quantum mechanics • Electrons orbit nucleus, but only certain orbits allowed • Angular momentum quantized: L = (mr)v = n(h/2π) n=1,2,3… • Fit mathematical prediction of H spectra by Balmer (1885) • Electrons in atoms cannot lose/gain energy co ...
... Bohr Atomic Model (1912) • Combined planetary model and quantum mechanics • Electrons orbit nucleus, but only certain orbits allowed • Angular momentum quantized: L = (mr)v = n(h/2π) n=1,2,3… • Fit mathematical prediction of H spectra by Balmer (1885) • Electrons in atoms cannot lose/gain energy co ...
ch18_LecturePPT - Chemistry at Winthrop University
... Hydrogen gas is fed to the anode. H2 molecules react chemically with the platinum catalyst and splits into two protons and two electrons. The PEM allows the protons through to the cathode. The electrons must travel through a circuit through an electric motor to get to the cathode. The protons and el ...
... Hydrogen gas is fed to the anode. H2 molecules react chemically with the platinum catalyst and splits into two protons and two electrons. The PEM allows the protons through to the cathode. The electrons must travel through a circuit through an electric motor to get to the cathode. The protons and el ...
Chapter 28: Quantum Physics
... 9.64 1021 J 0.060 eV (b) Through what potential difference should the electrons be accelerated to have this wavelength? ...
... 9.64 1021 J 0.060 eV (b) Through what potential difference should the electrons be accelerated to have this wavelength? ...
the bohr-sommerfeld model of the atom
... 4a. Overview. The Bohr model can be applied to two-particle atomic systems other than atomic hydrogen. For example, it can be applied to any hydrogen-like ion that consists of a single electron orbiting a nucleus containing more than one proton. Such a system is obtained by ionizing (removing) all b ...
... 4a. Overview. The Bohr model can be applied to two-particle atomic systems other than atomic hydrogen. For example, it can be applied to any hydrogen-like ion that consists of a single electron orbiting a nucleus containing more than one proton. Such a system is obtained by ionizing (removing) all b ...
Section1 Final Key
... is always lower than the classical energy. T / F: A spherical harmonic function Ylm (θ, φ) is an eigenfunction of the L̂2 operator with eigenvalue h̄2 l(l + 1). T / F : Any linear combination of solutions to the time independent Schrödinger equation is also a solution of that equation. T / F: The e ...
... is always lower than the classical energy. T / F: A spherical harmonic function Ylm (θ, φ) is an eigenfunction of the L̂2 operator with eigenvalue h̄2 l(l + 1). T / F : Any linear combination of solutions to the time independent Schrödinger equation is also a solution of that equation. T / F: The e ...
Key Concepts for Exam #2
... If the frequency of incident light is above the threshold frequency, then as the intensity of light increases, the kinetic energy of ejected electrons remains constant and the number of electrons increases. In addition, as the frequency of light increases, the kinetic energy of ejected electrons inc ...
... If the frequency of incident light is above the threshold frequency, then as the intensity of light increases, the kinetic energy of ejected electrons remains constant and the number of electrons increases. In addition, as the frequency of light increases, the kinetic energy of ejected electrons inc ...
phys_syllabi_412.pdf
... Topics to be covered: 1. Angular momentum and Spin; Addition of Angular Momenta (and Spin) 2. Charged particle in a Magnetic Field 3. Identical particles (Fermions, Bosons; Examples) 4. Time-independent and Time-dependent perturbation theory 5. Fermi’s Golden Rule 6. Variational Principle (Trial Wav ...
... Topics to be covered: 1. Angular momentum and Spin; Addition of Angular Momenta (and Spin) 2. Charged particle in a Magnetic Field 3. Identical particles (Fermions, Bosons; Examples) 4. Time-independent and Time-dependent perturbation theory 5. Fermi’s Golden Rule 6. Variational Principle (Trial Wav ...
Document
... energy state to another by absorbing or emitting photons of radiant energy of certain frequencies. He described the lines in the hydrogen spectrum as the energy given off when an electron in an excited state returns to the ground state. A flame or the application of high voltage imparts energy to th ...
... energy state to another by absorbing or emitting photons of radiant energy of certain frequencies. He described the lines in the hydrogen spectrum as the energy given off when an electron in an excited state returns to the ground state. A flame or the application of high voltage imparts energy to th ...
Lectures 3-5
... has a fixed set of allowed values). Only orbitals whose angular momentum is an integer multiple of h/2p are “allowed”. These orbitals are called stationary states. •The emission or absorption of light occurs when electrons ‘jump’ from one orbital to another. • Using these assumptions and basic physi ...
... has a fixed set of allowed values). Only orbitals whose angular momentum is an integer multiple of h/2p are “allowed”. These orbitals are called stationary states. •The emission or absorption of light occurs when electrons ‘jump’ from one orbital to another. • Using these assumptions and basic physi ...
Arrangement of Electrons in Atoms
... Effect: emission of electrons from a metal when light shines on the metal Video -13 ...
... Effect: emission of electrons from a metal when light shines on the metal Video -13 ...
stringtheory1s
... tried to understand them in terms of oldfashioned ideas … But at a certain point the old-fashioned ideas would begin to fail, so a warning was developed that said, in effect, ‘Your old-fashioned ideas are no damn good ...
... tried to understand them in terms of oldfashioned ideas … But at a certain point the old-fashioned ideas would begin to fail, so a warning was developed that said, in effect, ‘Your old-fashioned ideas are no damn good ...
MODERN PHYSICS CET questions from Bohr`s atom model
... 51. Pick out the incorrect statement from the following : 1. Strokes lines have wavelengths greater than that of the incident light 2. Strokes lines are more intense than the antistokes lines 3. The intensity of stokes lines is found to depend on temperature 4. Stokes and antistokes lines are polari ...
... 51. Pick out the incorrect statement from the following : 1. Strokes lines have wavelengths greater than that of the incident light 2. Strokes lines are more intense than the antistokes lines 3. The intensity of stokes lines is found to depend on temperature 4. Stokes and antistokes lines are polari ...
Ch. 2 - Ltcconline.net
... 3. Define a compound and explain how compounds in living organisms are different from compounds in nonliving things. 4. Describe the structure of an atom. 5. Distinguish between atomic number and atomic weight or mass number of an atom. 6. Define an isotope and explain what makes some isotopes radio ...
... 3. Define a compound and explain how compounds in living organisms are different from compounds in nonliving things. 4. Describe the structure of an atom. 5. Distinguish between atomic number and atomic weight or mass number of an atom. 6. Define an isotope and explain what makes some isotopes radio ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).