Chap30-DrJJ - 2 slides
... In the Bohr model, a photon is emitted when the electron drops from a larger, higherenergy orbit to a smaller, lower energy orbit. In a hydrogen atom, electrons’ total (kinetic & potential) energy can have only certain allowed values corresponding different orbits (stationary state or orbits) of the ...
... In the Bohr model, a photon is emitted when the electron drops from a larger, higherenergy orbit to a smaller, lower energy orbit. In a hydrogen atom, electrons’ total (kinetic & potential) energy can have only certain allowed values corresponding different orbits (stationary state or orbits) of the ...
Quantum Physics - The University of Sydney
... Describe qualitatively the differences between the energy levels, wave functions and probability density functions for finite and infinite one-dimensional potential wells. ...
... Describe qualitatively the differences between the energy levels, wave functions and probability density functions for finite and infinite one-dimensional potential wells. ...
A Brief History of Modern Physics and the development of the
... E(x,t). But this interpretation could not explain a host of experimental results, such as that fact that a particle with a large extended wave function is always found at one small spot when a position measurement is made. It was German theorist Max Born, who late in 1927 proposed that the wave func ...
... E(x,t). But this interpretation could not explain a host of experimental results, such as that fact that a particle with a large extended wave function is always found at one small spot when a position measurement is made. It was German theorist Max Born, who late in 1927 proposed that the wave func ...
Chapter 28
... • In 1913 Bohr provided an explanation of atomic spectra that includes some features of the currently accepted theory • His model was an attempt to explain why the atom was stable and included both classical and non-classical ideas ...
... • In 1913 Bohr provided an explanation of atomic spectra that includes some features of the currently accepted theory • His model was an attempt to explain why the atom was stable and included both classical and non-classical ideas ...
PHYS 305 - Modern Physics (Spring 2016) Department of Physics
... Modern Physics is a undergraduate level course which is intended for students, who have already studied introductory level physics. This course provides a basic introduction to better understanding of special relativity, Quantum mechanics, and applications of quantum theory to: atomic and molecular ...
... Modern Physics is a undergraduate level course which is intended for students, who have already studied introductory level physics. This course provides a basic introduction to better understanding of special relativity, Quantum mechanics, and applications of quantum theory to: atomic and molecular ...
History of Atomic theory
... tiny, positively charged nucleus which contains most of the mass. Most of the atom is filled with empty space occupied by electrons. B. This is the first proposed model of the atom. Matter is composed of indestructible, indivisible atoms. Different elements are composed of different particles. C. Hi ...
... tiny, positively charged nucleus which contains most of the mass. Most of the atom is filled with empty space occupied by electrons. B. This is the first proposed model of the atom. Matter is composed of indestructible, indivisible atoms. Different elements are composed of different particles. C. Hi ...
EP-307 Introduction to Quantum Mechanics
... three laws which apparently have no concern with each other describe one physical phenomenon? Why is it a physical phenomenon? Planck solved the mystery by enunciating that it emitted radiation in quantas of h ...
... three laws which apparently have no concern with each other describe one physical phenomenon? Why is it a physical phenomenon? Planck solved the mystery by enunciating that it emitted radiation in quantas of h ...
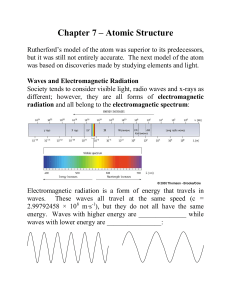
Lecture 8 1 Planck-Einstein Relation E = hν 2 Time evolution of real
... E = h̄ω where h̄ = h/2π. ν is linear frequency and ω is angular frequency. The fundamental constant h is called Planck’s constant and is equal to 6.62608 ×10−34 Js (h̄ = 1.05457 × 10−34 Js, or 1.05457 × 10−27 erg s). This relation was first proposed by Planck in 1900 to explain the properties of bla ...
... E = h̄ω where h̄ = h/2π. ν is linear frequency and ω is angular frequency. The fundamental constant h is called Planck’s constant and is equal to 6.62608 ×10−34 Js (h̄ = 1.05457 × 10−34 Js, or 1.05457 × 10−27 erg s). This relation was first proposed by Planck in 1900 to explain the properties of bla ...
ATOMS
... • This is the current model we use today & is more accurate. It says that electrons are found in a “cloud” around the nucleus (kind of like the spray of water from a sprinkler, each drop represents where an electron might be). • Electron Cloud Model Video (00:33) ...
... • This is the current model we use today & is more accurate. It says that electrons are found in a “cloud” around the nucleus (kind of like the spray of water from a sprinkler, each drop represents where an electron might be). • Electron Cloud Model Video (00:33) ...
South Pasadena • AP Chemistry Name
... The ionization energy of an element is the energy required to remove the most loosely held electron from atoms of the element in the gaseous state (cf. page 357). It is usually expressed in units of kJ/mol. Given that R, the Rydberg constant, is 1.097 x 107 m-1 h, Planck’s constant, is 6.626 x 10-34 ...
... The ionization energy of an element is the energy required to remove the most loosely held electron from atoms of the element in the gaseous state (cf. page 357). It is usually expressed in units of kJ/mol. Given that R, the Rydberg constant, is 1.097 x 107 m-1 h, Planck’s constant, is 6.626 x 10-34 ...
Collectively Moving Electrons
... electron from an upper shell. Upon its way through the high lying shells the decaying electron transfers its excessive energy to the electrons there (light blue shade) which can gain enough energy to leave the atom. A team of scientists using the high brilliant x-ray source PETRA III at DESY was abl ...
... electron from an upper shell. Upon its way through the high lying shells the decaying electron transfers its excessive energy to the electrons there (light blue shade) which can gain enough energy to leave the atom. A team of scientists using the high brilliant x-ray source PETRA III at DESY was abl ...
III. Quantum Model of the Atom
... C. Quantum Numbers Pauli Exclusion Principle No two electrons in an atom can have the same 4 quantum numbers. Each e- has a unique “address”: 1. Principal # 2. Ang. Mom. # 3. Magnetic # 4. Spin # ...
... C. Quantum Numbers Pauli Exclusion Principle No two electrons in an atom can have the same 4 quantum numbers. Each e- has a unique “address”: 1. Principal # 2. Ang. Mom. # 3. Magnetic # 4. Spin # ...
Ground State
... Many-body effect caused by electron-electron interaction Open shell atoms Hund’s rule: the ground state has the maximum total spin and maximum orbital moment ...
... Many-body effect caused by electron-electron interaction Open shell atoms Hund’s rule: the ground state has the maximum total spin and maximum orbital moment ...
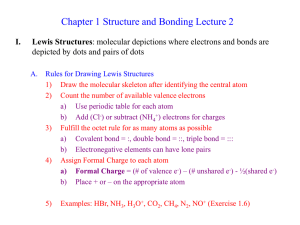
Ch 1 Lecture 2
... Major Resonance Contributor = contributes most to the composite structure a) Structures with the maximum octets are most important b) Charges should fit electronegativities c) Minimize charge separation d) Examples: NO+, Formaldehyde, Diazomethane, (Exercise 1-9) ...
... Major Resonance Contributor = contributes most to the composite structure a) Structures with the maximum octets are most important b) Charges should fit electronegativities c) Minimize charge separation d) Examples: NO+, Formaldehyde, Diazomethane, (Exercise 1-9) ...
Basic properties of atomic nuclei
... 43.4. Neutrons are placed in a magnetic field with magnitude 2.30 T. (a) What is the energy difference between the states with the nuclear spin angular momentum components parallel and anti parallel to the field? Which state is lower in energy, the one with its spin component parallel to the fleld o ...
... 43.4. Neutrons are placed in a magnetic field with magnitude 2.30 T. (a) What is the energy difference between the states with the nuclear spin angular momentum components parallel and anti parallel to the field? Which state is lower in energy, the one with its spin component parallel to the fleld o ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).