Quantum physics I
... to any change in the universe immediately, before measurement •Faster than light signaling- forbidden by special relativity •Gets around von Neumann’s proof- the electrons combine in “unreasonable ways” •Real fields moving faster than light = time travel, reversed causality ...
... to any change in the universe immediately, before measurement •Faster than light signaling- forbidden by special relativity •Gets around von Neumann’s proof- the electrons combine in “unreasonable ways” •Real fields moving faster than light = time travel, reversed causality ...
Adding Fermi-Dirac Statistics to the Drude Model = Sommmerfield
... wrong? In particular, the electrons don’t seem to be scattered by each other. Why? Why is the actual heat capacity of metals much smaller than predicted? ...
... wrong? In particular, the electrons don’t seem to be scattered by each other. Why? Why is the actual heat capacity of metals much smaller than predicted? ...
Week 14 Bellwork - Hobbs High School
... the nuclear charge is increasing with a consequently stronger attraction for electrons and an increase in ionization energy. -orAs you go across a period, the atomic radii decreases. The valence electrons are closer to the nucleus. Therefore, there will be a stronger attraction for electrons and an ...
... the nuclear charge is increasing with a consequently stronger attraction for electrons and an increase in ionization energy. -orAs you go across a period, the atomic radii decreases. The valence electrons are closer to the nucleus. Therefore, there will be a stronger attraction for electrons and an ...
Midterm Review Date
... Midterm Review 44. Which substance contains bonds that involved the transfer of electrons from one atom to another? A) CO 2 B) NH 3 C) KBr D) Cl 2 45. Based on bond type, which compound has the ...
... Midterm Review 44. Which substance contains bonds that involved the transfer of electrons from one atom to another? A) CO 2 B) NH 3 C) KBr D) Cl 2 45. Based on bond type, which compound has the ...
PDF Format - 1 slide per page
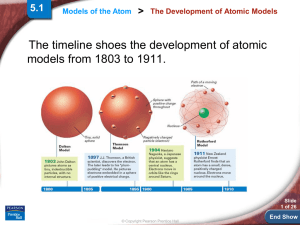
... • 1913 - Bohr model of the atom (very briefly!) stable orbits in which the electron can exist without radiating and thus not spiralling into the nucleus l (b (butt no th theoretical ti l jjustification) tifi ti ) each spectral line is due to energy lost when the electron falls from a higher to l ...
... • 1913 - Bohr model of the atom (very briefly!) stable orbits in which the electron can exist without radiating and thus not spiralling into the nucleus l (b (butt no th theoretical ti l jjustification) tifi ti ) each spectral line is due to energy lost when the electron falls from a higher to l ...
End Show
... What does the quantum mechanical model determine about the electrons in an atom? The quantum mechanical model determines the allowed energies an electron can have and how likely it is to find the electron in various locations Slide around the nucleus. 6 of 26 © Copyright Pearson Prentice Hall ...
... What does the quantum mechanical model determine about the electrons in an atom? The quantum mechanical model determines the allowed energies an electron can have and how likely it is to find the electron in various locations Slide around the nucleus. 6 of 26 © Copyright Pearson Prentice Hall ...
Atoms of different elements are
... conclusion that an atom is composed mostly of (A) empty space and has a small, negatively charged nucleus (B) empty space and has a small, positively charged nucleus (C) a large, dense, positively charged nucleus (D) a large, dense, negatively charged nucleus 12. Atoms of 16O, 17O, and 18O have the ...
... conclusion that an atom is composed mostly of (A) empty space and has a small, negatively charged nucleus (B) empty space and has a small, positively charged nucleus (C) a large, dense, positively charged nucleus (D) a large, dense, negatively charged nucleus 12. Atoms of 16O, 17O, and 18O have the ...
AMO-1: Table of Contents Fall 2004, C. D. Lin
... This part of the lecture is adopted from BJ--Appendix 5. Here are just a few key remarks. (i) The momentum space satisfies an integral eq. (A5.13). The potential becomes a kernel of the integral equation. (ii) For spherical potential, the momentum space wavefunction is separable, ...
... This part of the lecture is adopted from BJ--Appendix 5. Here are just a few key remarks. (i) The momentum space satisfies an integral eq. (A5.13). The potential becomes a kernel of the integral equation. (ii) For spherical potential, the momentum space wavefunction is separable, ...
atomic spectroscopy 2005
... deep understanding of atomic structure and molecular structure. Beyond this, modern techniques reveal further small corrections associated with the quantum theory of fields, and some of the most precise measurements in science In this experiment you will measure the Balmer series, the “spin-orbit” c ...
... deep understanding of atomic structure and molecular structure. Beyond this, modern techniques reveal further small corrections associated with the quantum theory of fields, and some of the most precise measurements in science In this experiment you will measure the Balmer series, the “spin-orbit” c ...
PHY201_MODULE_1_DOC - abuad lms
... A dramatic explanation of the Rydberg spectral expression resulted. Many modern ideas about atomic and molecular structure stem from this model. Bohr suggested the following postulate to explain the electron motion in an atom and the observed spectral lines: i. ...
... A dramatic explanation of the Rydberg spectral expression resulted. Many modern ideas about atomic and molecular structure stem from this model. Bohr suggested the following postulate to explain the electron motion in an atom and the observed spectral lines: i. ...
Time Evolution in Closed Quantum Systems
... equations, Liouville equation, etc.), which can present very different characteristics depending on which physical system they correspond to. From the beginning of the quantum theory, physicists have been often trying to translate the methods which were useful in the classical case to the quantum on ...
... equations, Liouville equation, etc.), which can present very different characteristics depending on which physical system they correspond to. From the beginning of the quantum theory, physicists have been often trying to translate the methods which were useful in the classical case to the quantum on ...
(a) n - Iust personal webpages
... 9-8 Interpreting and Representing the Orbitals of the Hydrogen Atom. ...
... 9-8 Interpreting and Representing the Orbitals of the Hydrogen Atom. ...
Atomic Physics - CAFE SYSTEM CANARIAS
... useful as an intuitive way of thinking about atomic structure and transitions between the energy levels. The ‘proper’ description in terms of atomic wavefunctions is presented in subsequent chapters. Before describing the theory of an atom with one electron, some experimental facts are presented. Th ...
... useful as an intuitive way of thinking about atomic structure and transitions between the energy levels. The ‘proper’ description in terms of atomic wavefunctions is presented in subsequent chapters. Before describing the theory of an atom with one electron, some experimental facts are presented. Th ...
From Last Time… - High Energy Physics
... A simple molecule consists of two protons and one electron orbiting around them. This molecule is A. Helium molecule B. Hydrogen molecule C. Lithium molecule ...
... A simple molecule consists of two protons and one electron orbiting around them. This molecule is A. Helium molecule B. Hydrogen molecule C. Lithium molecule ...
Density Matrix
... in which case ρ = |ψ >< ψ| if the state vector is normalized to unity. In summary, by the term “state of a system” we will understand as a state of a micro or macroscopic system defined by its complete density matrix. With that understanding, not all states are characterized by a state vector. Only ...
... in which case ρ = |ψ >< ψ| if the state vector is normalized to unity. In summary, by the term “state of a system” we will understand as a state of a micro or macroscopic system defined by its complete density matrix. With that understanding, not all states are characterized by a state vector. Only ...
Quantum Physics and Human Affairs
... quantized field is like water in a bucket that, for unknown reasons, must contain either exactly 1 gallon, or 2 gallons, or 3 gallons, etc. of water and never any intermediate amount such as 1.7 gallons. Such a "quantized bucket of water" could gain or lose water only in sudden 1-gallon increments o ...
... quantized field is like water in a bucket that, for unknown reasons, must contain either exactly 1 gallon, or 2 gallons, or 3 gallons, etc. of water and never any intermediate amount such as 1.7 gallons. Such a "quantized bucket of water" could gain or lose water only in sudden 1-gallon increments o ...
Research Statement
... 2D optical lattice, and furthermore, they can even perform “spin” flips on individual atoms! If we can successfully simulate the dynamics of such a system using Chebyshev methods, we will be very close to designing a quantum computer. In general, I am fascinated by emergent phenomena, from new types ...
... 2D optical lattice, and furthermore, they can even perform “spin” flips on individual atoms! If we can successfully simulate the dynamics of such a system using Chebyshev methods, we will be very close to designing a quantum computer. In general, I am fascinated by emergent phenomena, from new types ...
Lecture 5
... Here, Rule 2: "The total energy of all electrons is minimum for atomic ground state" comes into play. Parallel spin wave function is symmetric and corresponding spatial wave function is antisymmetric. Antisymmetric spatial wave function describes an electron distribution where electrons are further ...
... Here, Rule 2: "The total energy of all electrons is minimum for atomic ground state" comes into play. Parallel spin wave function is symmetric and corresponding spatial wave function is antisymmetric. Antisymmetric spatial wave function describes an electron distribution where electrons are further ...
the hydrogen spectrum and shells
... In 1913, Niels Bohr introduced his model of the hydrogen atom. He assumed that the electron within the hydrogen atom will not absorb or radiate energy so long as it stays in one of a number of circular orbits. His model of the atom was designed to explain the observation that the electromagnetic rad ...
... In 1913, Niels Bohr introduced his model of the hydrogen atom. He assumed that the electron within the hydrogen atom will not absorb or radiate energy so long as it stays in one of a number of circular orbits. His model of the atom was designed to explain the observation that the electromagnetic rad ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).