Angular momentum
... Let us assume that the operators (Lx , Ly , Lz ) ≡ L which represent the components of orbital angular momentum in quantum mechanics can be defined in an analogous manner to the corresponding components of classical angular momentum. In other words, we are going to assume that the above equations sp ...
... Let us assume that the operators (Lx , Ly , Lz ) ≡ L which represent the components of orbital angular momentum in quantum mechanics can be defined in an analogous manner to the corresponding components of classical angular momentum. In other words, we are going to assume that the above equations sp ...
Free-fall time
... What would the magnetic field have to be to equal that pressure (in Gauss)? Pm =B2/2m0, B = (2m0Pm)½ = [(2)(4pX107)(1.38X10-6)]½ = 1.86X10-6 T = 0.02 G ...
... What would the magnetic field have to be to equal that pressure (in Gauss)? Pm =B2/2m0, B = (2m0Pm)½ = [(2)(4pX107)(1.38X10-6)]½ = 1.86X10-6 T = 0.02 G ...
Post-doctoral position in ultracold atomic physics Laboratoire de
... Building on the expertise of our group on large spin magnetism driven by dipole-dipole interactions in chromium gases, we envision to study quantum magnetism of large spin fermions using strontium atoms. Our experiment will allow the measurement of each of 10 spin states with single-site resolution ...
... Building on the expertise of our group on large spin magnetism driven by dipole-dipole interactions in chromium gases, we envision to study quantum magnetism of large spin fermions using strontium atoms. Our experiment will allow the measurement of each of 10 spin states with single-site resolution ...
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
... Note that you will have to perform three uncoupledcoupled transformations: ...
... Note that you will have to perform three uncoupledcoupled transformations: ...
Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... have an “element of reality” i.e. a clear inherent feature defining their independent x spin projection. Let us call these 3 independent characters who can take the value of +/-1: m1x, m2x, and m3x. However, as can be seen from (1) the same values can also be know by measuring the y projection of th ...
... have an “element of reality” i.e. a clear inherent feature defining their independent x spin projection. Let us call these 3 independent characters who can take the value of +/-1: m1x, m2x, and m3x. However, as can be seen from (1) the same values can also be know by measuring the y projection of th ...
The Halo at the Centre of the Atom
... • Nuclei do not obey the laws of ordinary matter, • But the peculiar laws of Quantum Mechanics, which govern atoms and all they contain. • Nuclei exhibit a unique range of quantum ...
... • Nuclei do not obey the laws of ordinary matter, • But the peculiar laws of Quantum Mechanics, which govern atoms and all they contain. • Nuclei exhibit a unique range of quantum ...
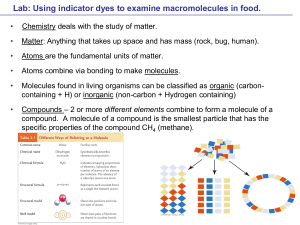
Chemistry B11 Chapter 2 Atoms Atoms:
... Mixture: is a combination of two or more pure substances. Mixtures are divided into two groups: 1- Homogeneous: the mixture is uniform and throughout and no amount of magnification will reveal the presence of different substances (for example, air and salt in water). 2- Heterogeneous: the mixture is ...
... Mixture: is a combination of two or more pure substances. Mixtures are divided into two groups: 1- Homogeneous: the mixture is uniform and throughout and no amount of magnification will reveal the presence of different substances (for example, air and salt in water). 2- Heterogeneous: the mixture is ...
Slide 1
... = s, p, d, f, g, h, .......(n-1) The symbol for the magnetic quantum number is m which defines the orbital. m = - , (- + 1), (- +2), .....0, ......., ( -2), ( -1), The last quantum number is the spin quantum number which has the symbol m s which characterizes the single electron. The s ...
... = s, p, d, f, g, h, .......(n-1) The symbol for the magnetic quantum number is m which defines the orbital. m = - , (- + 1), (- +2), .....0, ......., ( -2), ( -1), The last quantum number is the spin quantum number which has the symbol m s which characterizes the single electron. The s ...
Day 19: Electrostatic Potential Energy & CRT Applications
... • Work Done to Dissemble a Hydrogen Atom • Cathode Ray Tube (CRT) Applications ...
... • Work Done to Dissemble a Hydrogen Atom • Cathode Ray Tube (CRT) Applications ...
Define the Scientific Method
... A) Atoms have an atomic nucleus B) Atoms have a negative charge C) Atoms have a mass D) Both B and C. _________ 5) What did the gold foil experiment prove? A) Atoms have an atomic nucleus B) The nucleus of an atom has a positive charge C) Atoms are mostly empty space D) All of the above. _________ 6 ...
... A) Atoms have an atomic nucleus B) Atoms have a negative charge C) Atoms have a mass D) Both B and C. _________ 5) What did the gold foil experiment prove? A) Atoms have an atomic nucleus B) The nucleus of an atom has a positive charge C) Atoms are mostly empty space D) All of the above. _________ 6 ...
2.1 Historical Development
... Ernest Rutherford directed a narrow beam of alpha particles obtained from polonium at an extremely thin sheet of a metal like silver and gold. After passing through the metal sheet, the alpha particles were made to strike a fluorescent screen. Rutherford`s observations were 1. Most of the alpha part ...
... Ernest Rutherford directed a narrow beam of alpha particles obtained from polonium at an extremely thin sheet of a metal like silver and gold. After passing through the metal sheet, the alpha particles were made to strike a fluorescent screen. Rutherford`s observations were 1. Most of the alpha part ...
semester ii
... Basics of Quantum Mechanics (14 Hrs) Stern - Gerlach experiment leading to vector space concept, Dirac notation for state vectorsket space, bra space, inner products – algebraic manipulation of operators – unitary operators, eigenkets and eigenvalues –Hermitian operators-concept of complete set-repr ...
... Basics of Quantum Mechanics (14 Hrs) Stern - Gerlach experiment leading to vector space concept, Dirac notation for state vectorsket space, bra space, inner products – algebraic manipulation of operators – unitary operators, eigenkets and eigenvalues –Hermitian operators-concept of complete set-repr ...
Exchange and Sign-change: The Pauli Exclusion Principle
... that electrons carry a 'spin', or intrinsic magnetic moment,apart from their orbital moment. Around that time, too, Pauli suggested his 'exclusion principle': two electrons in an atom cannot have all their quantum numbers the same, and if they occupy the same orbital, they must have different spin. ...
... that electrons carry a 'spin', or intrinsic magnetic moment,apart from their orbital moment. Around that time, too, Pauli suggested his 'exclusion principle': two electrons in an atom cannot have all their quantum numbers the same, and if they occupy the same orbital, they must have different spin. ...
Chapter 1: Atomic Structure
... Wolfgang Pauli helped develop quantum mechanics in the 1920s by developing the concept of spin and the Pauli exclusion principle, which states that if two electrons occupy the same orbital, they must have different spin (intrinsic angular momentum). This principle has been generalized to other quant ...
... Wolfgang Pauli helped develop quantum mechanics in the 1920s by developing the concept of spin and the Pauli exclusion principle, which states that if two electrons occupy the same orbital, they must have different spin (intrinsic angular momentum). This principle has been generalized to other quant ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).