Handout - UNT Chemistry
... No Required Text: When I've taught CHEM 5210 in the past, students commented that they utilized the PP lectures, homework, old exams to study for tests, and didn't find the textbook particularly useful. Therefore, I do not assign any required text for the course. I would suggest that as we reach spe ...
... No Required Text: When I've taught CHEM 5210 in the past, students commented that they utilized the PP lectures, homework, old exams to study for tests, and didn't find the textbook particularly useful. Therefore, I do not assign any required text for the course. I would suggest that as we reach spe ...
Dept. d`Enginyeria Electrònica, Universitat Autònoma de Barcelona
... The adaptation of Bohmian mechanics to electron transport leads to a quantum Monte Carlo (MC) algorithm, where randomness appears because of the uncertainties in energies, initial positions of (Bohmian) trajectories, etc [2-4]. The ability of our simulator to deal with strongly correlated systems is ...
... The adaptation of Bohmian mechanics to electron transport leads to a quantum Monte Carlo (MC) algorithm, where randomness appears because of the uncertainties in energies, initial positions of (Bohmian) trajectories, etc [2-4]. The ability of our simulator to deal with strongly correlated systems is ...
Derivation of the Pauli Exclusion Principle
... the commutators [3]. Calculate a change in the azimuthal quantum number l when the smaller circle or one of identical two circles emits one entanglon (since in this paper is j ≥ k so there is the transition k k – 1) whereas the second circle in the pair almost simultaneously absorbs the emitted en ...
... the commutators [3]. Calculate a change in the azimuthal quantum number l when the smaller circle or one of identical two circles emits one entanglon (since in this paper is j ≥ k so there is the transition k k – 1) whereas the second circle in the pair almost simultaneously absorbs the emitted en ...
Introduction to Quantum Mechanic
... localization. If E=constant, this is a stationary state, independent of t which is not defined. ...
... localization. If E=constant, this is a stationary state, independent of t which is not defined. ...
Presentation - University of Colorado Boulder
... Use superposition to calculate 2n values of function simultaneously and do not read out the result until a useful outout is expected with reasonably high probability. Use entanglement: measurement of states can be highly correlated ...
... Use superposition to calculate 2n values of function simultaneously and do not read out the result until a useful outout is expected with reasonably high probability. Use entanglement: measurement of states can be highly correlated ...
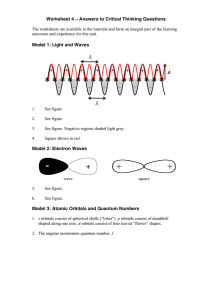
Answers to Critical Thinking Questions 4
... The 2s has one radial node and the 3s has two radial nodes. 3p have one radial node. In general, the number of radial nodes is equal to n – l - 1. ...
... The 2s has one radial node and the 3s has two radial nodes. 3p have one radial node. In general, the number of radial nodes is equal to n – l - 1. ...
Ch8lsn22Chem105
... For an excited hydrogen atom with the quantum number n equal to 9, which of the following statements is true? A) The energy of the atom is less than the energy for the state in which n is equal to 8. B) If ℓ = 0, there are nine possible values for the magnetic quantum number mℓ. C) The smallest valu ...
... For an excited hydrogen atom with the quantum number n equal to 9, which of the following statements is true? A) The energy of the atom is less than the energy for the state in which n is equal to 8. B) If ℓ = 0, there are nine possible values for the magnetic quantum number mℓ. C) The smallest valu ...
Slides from Lecture 9-11
... Often we’re interested in quantum numbers, not the wave pattern: vector approach avoids calculating wave functions when not needed. Wave function picture incomplete: If you know ψ(r) you know everything about: position, momentum, KE, orbital angular momentum …but nothing about spin (+ other mo ...
... Often we’re interested in quantum numbers, not the wave pattern: vector approach avoids calculating wave functions when not needed. Wave function picture incomplete: If you know ψ(r) you know everything about: position, momentum, KE, orbital angular momentum …but nothing about spin (+ other mo ...
Document
... Atom is mostly empty space with a small (r = 10-15 m) positively charged nucleus surrounded by “cloud” of electrons (r = 10-10 m) Physics 102: Lecture 24, Slide 9 ...
... Atom is mostly empty space with a small (r = 10-15 m) positively charged nucleus surrounded by “cloud” of electrons (r = 10-10 m) Physics 102: Lecture 24, Slide 9 ...
QM-01
... The Heisenberg’s uncertainty principle Sets limit on what we can observe. Consider our attempt to view the electrons in the double slit system by shining light of wavelength λ on them with photon momentum pphoton = h/λ . If we manage to see an electron it will be because one of these photons has st ...
... The Heisenberg’s uncertainty principle Sets limit on what we can observe. Consider our attempt to view the electrons in the double slit system by shining light of wavelength λ on them with photon momentum pphoton = h/λ . If we manage to see an electron it will be because one of these photons has st ...
Hydrogen 1
... state energy level of the hydrogen atom in the Bohr Model! While equation (23) is the Bohr radius of the atom! We have now obtained these parameters from the Schrodinger equation! What do the full solutions of the radial equation look like, (the assiciated Laguerre functions). Again they are polynom ...
... state energy level of the hydrogen atom in the Bohr Model! While equation (23) is the Bohr radius of the atom! We have now obtained these parameters from the Schrodinger equation! What do the full solutions of the radial equation look like, (the assiciated Laguerre functions). Again they are polynom ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).