* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Group A_lipid - UniMAP Portal
Survey
Document related concepts
Lipopolysaccharide wikipedia , lookup
Epoxyeicosatrienoic acid wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Cholesterol wikipedia , lookup
Low-density lipoprotein wikipedia , lookup
High-density lipoprotein wikipedia , lookup
Ethanol-induced non-lamellar phases in phospholipids wikipedia , lookup
Phospholipid-derived fatty acids wikipedia , lookup
Transcript
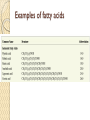
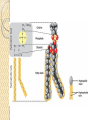
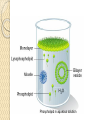
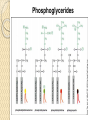
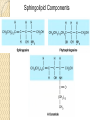
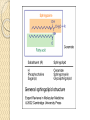
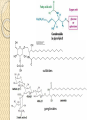
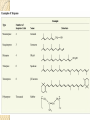
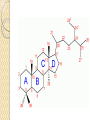
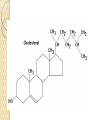
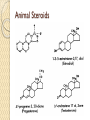
PTT103 BIOCHEMISTRY LIPID Pn Khadijah Hanim Abdul Rahman School of Bioprocess Engineering Week 5: 8/10/2012 Sem 1, 2012/2013 Course outcome Able to differentiate basic structure, properties, functions and classification of important biomolecules. Content: - Structure and function of lipids and their derivatives - Classification of lipids Outline Lipid Classes - Fatty acids and their derivatives - Triacylglycerols - Wax esters - Phospholipids - Sphingolipids - Isoprenoids Membranes - Membrane structure - Membrane function Introduction diverse group of biomolecules eg. Fats, oils, phospholipids, steroids, carotenoids- which differ in structure and function are considered as lipids Lipids – Those substances from living organisms that dissolve in nonpolar solvents eg. Ether, chloroform, acetone but not in water. Role & function as : ◦ structural components in cell membranes (e.g phospolipids and sphingolipids) ◦ Fats and oil means to store energy (e.g triacylglycerols) ◦ chemical signals, vitamins, or pigments, ◦ protective molecules (outer coatings for cells). Lipid classes Lipids may be classified into following classes: Fatty acids and their derivatives Triacylglycerols Wax esters Phospholipids Sphingolipids Isoprenoids Fatty acids and their derivatives Fatty acids – monocarboxylic acids that contain hydrocarbon chains of variable length (12-20 C), R-COOH Fatty acids are important components of several types of lipid molecules Occur primarily in triacylglycerols and several types of membrane bound lipid molecules. Fatty acids and their derivatives Naturally occurring fatty acids have an even no of C atoms that form unbranched chain. 2 types saturated (only carbon-carbon single bond) unsaturated (one/more double bonds) - can occur in two isomeric forms; cis/trans - cis : identical groups are on the same side of a double bond - Trans : identical groups are on opposite sides of a double bond Cis-isomers : Both R groups are on the same side of the carbon-carbon double bond Trans-isomers : Have R groups on different sides. Monounsaturated : 1 double bond Polyunsaturated : > 1 double bonds Fatty acid structure Naturally occurring FA are in cisconfiguration The presence of cis double bond causes ‘kink’ in FA chain Thus, unsaturated FA do not pack closely together as saturated FA. Less energy is required to disrupt the intermolecular forces between unsaturated FA- lower melting point Examples of fatty acids number of double bonds. position of a double bond Tot number of C Fatty acid with one double bond are referred to as monounsaturated molecules When two or more double bonds occur in FA usually separated by methyl grouppolyunsaturated. Plants & bacteria synthesize all fatty acids they need from acetyl-CoA. Mammals can synthesize saturated & some monounsaturated fatty acid. Other unsaturated FA obtain from dietary source. Nonessential FA – can be synthesized Essential FA – eg: linoleic and linolenic acids are obtained from diet (vege oils,nuts,seeds) Linoleic and linolenic acids: membrane structure, precursors for several important metabolites. Symptoms of low-fat diets – deficient in essential FA: Dermatitis (scally skin) Poor wound healing Reduced resistant to infection Hair loss Thrombocytopenis (reduction in no of platelets) Triacylglycerols Ester of glycerol with 3 fatty acids molecules Neutral fats – no charge Most contain FA of varying lengths, which may be saturated, unsaturated or a combination of both Referred as fats or oils depend on FA composition Fats – solid at room temp, mostly saturated FA Fats – solid at room temp, mostly saturated FA Oils – liquid at room temp, high unsaturated FA In animals triacylglycerols (fats) - store energy > efficiently than glycogen: 1. TAGs are hydrophobic, they coalesce into compact, anhydrous droplets within cells. Adipocyte stores TAG. - Glycogen binds to water- the anhydrous TAG store equivalent amount of energy in about 1-8th of glycogen vol. 2. TAG are less oxidized than carbohydrate. TAG release more energy when they are degraded. provide insulation at low temp- poor conductor of heat. Adipose tissue prevent the heat loss. In plants triacylglycerols (oils) - energy reserve in fruits and seeds - high amounts of unsaturated FA- plant oils (eg oleic & linoleic) soybean, peanut, olive Wax esters are esters formed from fatty acids and long chain alcohols Nonpolar lipid Function – protective coating on leaves, stems, fruits, skin and fur of animals carnauba wax produced by the leaves of Brazilian wax palm – 32C carboxylic acid & 34C alcohol component. Beeswax – 26C carboxylic acid & 30C alcohol component Phospholipids Roles : 1) Structural components of membranes 2) Emulsifying agents 3) Surface active agents (substance that lowers surface tension of a liquid) Amphipathic molecule Have hydrophobic and hydrophilic domains Hydrophobic domain - composed of hydrocarbon chains of fatty acids Hydrophilic domain (polar head group) - composed of phosphate & other charged or polar group Suspended in water they spontaneously rearrange into ordered structures ◦ Hydrophobic group exclude water ◦ Hydrophilic group exposed to water (Next slide) ◦ They form bimolecular layers: (Basis of membrane structure) Phospholipid in aqueous solution 2 types phospholipids : phosphoglycerides – mol contain glycerol, fatty acids, phosphate, alcohol (eg choline). Found in cell membrane Sphingomyelins – contain sphingosine instead of glycerol, fatty acids, phoshate, alcohol (classified as sphingolipid) – discuss later Phosphoglycerides The simplest phosphoglyceridephosphotidic acid (precursor for all phosphoglyceride molecules). Phosphatidic acid is composed of glycerol-3-phosphate that is esterified with 2 FAs. O O H2C R 2 CO O R CH H2C O O P O O X - Basic Structure of phosphoglyceride Sphingolipids Important membrane components of animal & plant membranes Contain long-chain amino alcohol (either sphingosine or phytosphingosine) linked to fatty acid mol by amide bond 3 subclasses – ceramide (core of sphingolipid), sphingomyelin (found in animal cells), glycosphingolipid Sphingolipid Components Sphingomyelin – animal cell membrane: found in greatest abundance in myelin sheath of nerve cells. - have a phosphorylcholine or phosphoethanolamine molecule with an ester linkage to the 1-hydroxy group of a ceramide. Ceramide are also precursors for glycolipids or refered as glycosphingolipid - In glycolipids: monosaccharide, disaccharide and oligosaccharide is attached to ceramide thru Oglycosidic linkage. - Glycolipids differ from sphingomyelin: contain no phosphate. Classes :- Cerebrosides have a single glucose or galactose at the 1-hydroxy position - Sulfatides are sulfated cerebrosides - Gangliosides sphingolipids that possess oligosaccharide groups, one of which must be sialic acid sulfatides gangliosides Isoprenoids Biomolecules contain repeating 5 carbon structural units (isoprene units) isoprene Biosynthetic pathway begin with formation of isopentenyl pyrophosphate from acetyl-CoA Consist of terpenes and steroids - - Terpenes (enormous group of molecules that are found largely in essential oils of plants) Classified according to number of isoprene residues they contain : Monoterpenes (2 isoprene units- 10 Cs) eg. geraniol in oil of geranium Sesquiterpenes (3 isoprenes) eg. Farnesene (part of citronella oilused in soaps and perfumes) Diterpenes (4 isoprenes) eg. Phytol, a plant alcohol - Triterpenes (6 isoprene) eg. Squalene in shark liver oil, olive oil - Tetraterpenes (8 isoprene) eg. Carotenoids, orange pigment - Polyterpene (Thousands isoprene) eg. Rubber (3000-6000 isoprene) - - - Steroids (derivatives of the hydrocarbon ring system of cholesterol) Complex derivatives of triterpenes (6 Cs) Eukaryotes & some bacteria Composed of 4 fused rings Distinguished from each other by placement of carbon-carbon double bonds and various constituents (OH, Carbonyl & alkyl groups) Eg cholesterol, progesterone, testosterone, estradiol - - - Cholesterol Important mol in animals cell membrane & precursor for synthesis of all steroid hormones, vit D & bile salts. Possesses 2 methyl (C-18 & C-19), attached to C-13 & C-10 & a double bond Has a OH group (sterol) Cholesterol often stored in the cells as a fatty acid ester. The esterification reaction is catalyzed by the enzyme acyl-CoA acyltransferase. Animal Steroids LIPOPROTEINS Lipoproteins- describe the protein that is covalently linked to lipid groups Commonly found in the blood plasma of mammals. Plasma lipoproteins transport lipid molecules (TAG, phospholipids & cholesterol) thru the bloodstream from 1 organ to the other. Protein components of lipoprotein- apoprotein Lipoproteins are classified according their density Types of lipoproteins - - - Chylomicrons- large lipoproteins of extremely low density. Transport dietary TAG and cholesteryl esters from intestine to muscle and adipose tissues. Very low density lipoproteins (VLDL)- synthesized in the liver, transport lipids to tissues. As VLDL are transported thru the body, they become depleted of TAGs and some apoprotein and phospholipids. Eventually, the TAG-depleted VLDL remnants are either picked up by the liver or converted to LDL. LDL carry cholesterol to tissues. LDL are engulfed by cells after binding to LDL receptors. - - High-density lipoprotein (HDL)- also produced in liver. Cholesteryl esters are formed when the plasma enzyme lecithin:cholestero acyltransferase transfers a FA residue from lecithin to cholesterol. HDL transport these cholesteryl ester to liver. Liver can dispose cholesterol, convert most of it to bile salts. Atheroscelerosis Chronic disease in which soft masses/plague accumulate on the inside of arteries. During plaque formation- smooth muscle cells, macrophages and various cell debris built up. As they are filled with lipid- they take a foam like appearance. Eventually, the plaque may calcify and protrude sufficiently into arterial lumens that blood flow impeded. Common consequences of atherosclerosiscoronary artery disease- damages heart muscle. Most of the cholesterol found in plaque is obtained by the ingestion of LDL by foam cells- directly correlated with high risk for coronary heart disease. High plasma HDL- low risk for coronary artery disease. Liver cells are the only cells that possess HDL receptors. Questions Classify and differentiate lipid classes What role do plasma lipoproteins play in human body? Why do plasma lipoproteins require a protein component to accomplish their role?