* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download cell respiration wilk hl ibdp
Biosynthesis wikipedia , lookup
Mitochondrion wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Photosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Phosphorylation wikipedia , lookup
Microbial metabolism wikipedia , lookup
Electron transport chain wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Citric acid cycle wikipedia , lookup
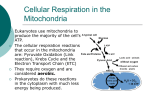
CELL RESPIRATION C6H12O6 + 6O2 --- 6H2O + 6CO2 + 36 ATP Humans use 1,000,000 molecules of ATP/cell/second !!!! Is this a CATABOLIC or ANABOLIC Reaction? REACTIONS OXIDATION • LOSS of electrons from a substance • Addition of oxygen atoms • Removal of hydrogen atoms REDUCTION • GAIN of electrons to a substance • Removal of oxygen atoms • Addition of hydrogen atoms RESPIRATION GLUCOSE FATTY ACIDS AMINO ACIDS OXIDATION GLYCOLYSIS •IF THE RESPIRATORY SUBSTRATE IS GLUCOSE THEN THE FIRST STAGE OF CELLULAR RESPIRATION IS GLYCOLYSIS •THIS PATHWAY OCCURS IN THE CYTOPLASM •LESS AMOUNT OF ENERGY IS PRODUCED •PARTIAL OXIDATION OF GLUCOSE OCCURS, AND DOES NOT REQUIRE OXYGEN •IT OCCURS IN BOTH AEROBIC AND ANAEROBIC RESPI RATION. •IT OCCURS IN BOTH PROKARYOTES & EUKARYOTES STEPS INVOLVED IN GLYCOLSIS STEP I PHOSPHORYLATION • 2PO4 groups are added to a GLUCOSE molecule to form HEXOSE BIPHOSPHATE. • 2ATP molecules provide the PO4 • Energy level of the hexose formed is raised by phosphorylation and this makes the subsequent reactions possible 2 ATP GLUCOSE 2 ADP HEXOSE BIPHOSPHATE STEP II: LYSIS • Each HEXOSE BIPHOSPHATE splits to form 2 molecules of TRIOSE PHOSPHATE . HEXOSE BIPHOSPHATE 2 molecules TRIOSE PHOSPHATE STEP III: OXIDATION of Triose phosphate 2 NAD+ 2 molecules of TRIOSE PHOSPHATE 2 NADH + H+ 3 CARBON COMPOUND carrying 2PO4 groups each STEP IV: ATP formation 4 ADP Two 3 CARBON COMPOUND formed 4 ATP 2 PYRUVATE MOLECULES Enzymes remove the 2 phosphate groups and provide them to ADP for ATP formation STEPS INVOLVED IN GLYCOLSIS STEP I: PHOSPHORYLATION STEP II: LYSIS STEP III: OXIDATION of Triose phosphate 2 NAD+ STEP IV: ATP formation 2 NADH + H+ 2 triose phosphate (3c) molecules glucose 2 ATP 2 ADP 2 INTERMEDIATE (3c) molecules 4 ADP Hexose biphosphate (6c) 4 ATP 2 pyruvate molecules • The fate of Pyruvate is decided by the availability of oxygen. • This step occurs only if oxygen is not available or is in short supply; ie . ANAEROBIC RESPIRATION In plants Each molecule of PYRUVATE CO2 Ethanol (2 C) COMPOUND In animals Each molecule of PYRUVATE(3C) Lactic acid (3 C) COMPOUND In animals Each molecule of PYRUVATE(3C) LINK REACTION Lactic acid (3 C) COMPOUND LINK REACTION • Pyruvate passes from the cytosol to the inner mitochondrial matrix by active transport • This step occurs only if oxygen is available; ie . AEROBIC RESPIRATION NAD+ NADH + H+ 2 CARBON COMPOUND ACETYL CoA Each molecule of PYRUVATE CoA CO2 • DeCarboxylation and Oxidation occur simultaneously hence the step is called Oxidative decarboxylation • Pyruvate + CoA forms Acetyl CoA • CoA comprises of [ adenine + ribose sugar + Pantothenic acid] • CoA is a carrier for Acetyl group into the Krebs cycle. NAD+ NADH + H+ Each molecule of PYRUVATE CoA 2 CARBON COMPOUND ACETYL CoA CO2 Link reaction summary Oxidation phosphorylation • The energy stored in NADH is used to generate a proton gradient across the inner membrane. • The energy of the proton gradient is used to make ATP (phosphorylate). • Glucose on oxidation during glycolysis and Krebs cycle , the Co-enzymes NAD and FAD are reduced to NADH + H+ & FADH + H+ • In the mitochondrial matrix electrons from NADH are transferred to Co Q by NADH DEHYDROGENASE; energy is released • As a result the H+ ions ( protons) are transferred to the inter membrane space. • Co Q carries the electrons to cytochrome bc1 complex ; energy is released • Electrons are carried forward from cytochrome bc1 complex to cytochrome c ; energy is released • As a result the more and more H+ ions ( protons) are transferred to the inter membrane space. • In the mitochondrial matrix electrons from FADH are transferred to Co Q; energy is released • As a result the H+ ions ( protons) are transferred to the inter membrane space. • Co Q carries the electrons to cytochrome bc1 complex ; energy is released • Electrons are carried forward from Cytochrome C to Cytochrome c oxidase; energy is released • As a result the more and more H+ ions ( protons) are transferred to the inter membrane space. Cytochrome c oxidase ultimately transfers electrons to Oxygen (terminal e acceptor) and water is formed as an end product. • Transfer of protons to the inter membrane space develops a proton motive force across the membrane. • Inner membrane is impermeable to protons so protons can pass through into the matrix is only through the ATP Synthase enzyme. Energy derived from the movement of these protons back into the inner matrix is used to synthesize ATP from ADP This is oxidative phosphorylation. Respiration chemiosmosis • Involves an electron transport chain in the membrane s of the cristae • Energy is released when electrons are exchanged from 1 carrier to another • Released energy is used to actively pump hydrogen ions into the inter-membrane space • Hydrogen ions come from the matrix • H ions diffuse back into the matrix through the channels of ATP synthase • ATP synthase catalyses the oxidative phosphorylation of ADP to ATP