* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Applying Proteomics in Biomedical Research
Survey
Document related concepts
G protein–coupled receptor wikipedia , lookup
Magnesium transporter wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein moonlighting wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Phosphorylation wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Protein structure prediction wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Chemical biology wikipedia , lookup
Proteolysis wikipedia , lookup
Western blot wikipedia , lookup
Transcript
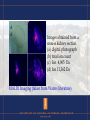
Using Proteomics for Biomedical Research Proteomics: coined by Wilkins and Williams …. The study of proteins as an result from genomics…………… Ruedi Abersold …defining the mandate of proteomics : “proteomics represents the effort to establish the identities, quantities, structures and biochemical and cellular functions of all proteins in an organism, organ, or organelle, and how these properties vary in space, time and physiological state.” Examples of Proteomes: ribosomes yeast nucleosomes transcriptional complexes biopsy samples affinity – chemical or biological interactions – IP etc. 2-D gel rat brain homogenate 1 mg protein load Resolved over 2000 spots pI and MW of protein spots 2-D gel of an uncharacterized bacteria collected from Yellowstone National Park Mammoth Hotsprings October 2007 (Collaboration with Bruce Fouke, UIUC Geology) Interpreting 2-D gel images •Image warping •Normalization •Statistical significance •Real spots versus artifacts •Internal standards •Gel to gel variations •Sensitivity “You’ve got one protein missing …” “No, you’ve one extra protein !” Human Genome Nucleic Acids • 30,000 genes • DNA is localized in the nucleus, simple extraction ! Proteins – extraction is always a challenge ! • localization (compartmentalization e.g. nucleus) • solubility, salt, pH More on protein extraction challenges: •Membrane proteins (hydrophobic) •Glycoproteins •Timing of protein extraction (e.g. cyclins) •Active versus inactive forms •Isozymes •Post-translational modifications (over 300) Protein abundance in cell varies by 5-6 orders !! Bruker Daltonics Fractionation Protein fractionation and purification is a key step in proteomics studies •Whole animal › organ › tissue › laser captured cell clusters…. •Whole cell extract › nuclear or cytoplasmic extract •Whole brain › hippocampus › post-synaptic membranes •Avoid protein degradation (proteolysis inhibitors) •For studies involving PTMs (e.g. phosphorylation use phosphatase inhibitors) •Chromatography (ion exchange, HPLC, affinity…) •Avoid inadvertent chemical modifications (carbamate, formyl or acetyl from acid treatment…….. etc) Common techniques for proteomics •Fractionation and isolation (extraction, centrifugation, chromatography) •Classical methods such as Edman Sequecing, amino acid analysis •Electrophoresis •Immunological methods – antibodies pull-down (IP), ELISA, western blot, immuno-histology, bead-based Luminex assays…… •Structural methods, scattering, microscopy, NMR •Gel based versus chomatography versus mass spec •Spectroscopy (VIS/UV), Mass spectrometry Fractionation (Agilent method) Agilent Beckman PF-2D • First dimension - chromatofocusing • Chromatofocusing is a column based chromatography method separation proteins according to their pI (similar to 2-D gel) • Second dimension is RP-HPLC • This method is non-gel based Functional Specific Protein Stains • • • • • • • Invitrogen (Molecular Probes) Pro-Q Diamond – phosphorylation Pro-Q-Emerald – glycosylation Quantitative, 3 orders Good sensitivity Works well with SYPRO-Ruby Compatible with mass spec DIGE • Fluorescence Difference in Gel electrophoresis • Protein samples labeled with CyDyes • Cy3 for Sample 1 • Cy5 for Sample 2 • Cy2, normalization, Samples 1 and 2 • All three labeled samples pooled and run in the same gel Cy3 channel Cy5 Channel Channel Cy3 and Cy5 superimposed Mass spectrometry to Proteomics is like PCR to Genomics 2002 Nobel Prizes in Chemistry Mass spectrometry for macromolecules "for their development of soft desorption ionisation methods for mass spectrometric analyses of biological macromolecules" Koichi Tanaka John B. Fenn MALDI ToF MS Matrix Assisted Laser Desorption Ionization Time-offlight mass spectrometer ESI MS Electro-Spray-Ionization mass spectrtometer Schematic of a modern MALDI-ToF MS using DHB as matrix Matrix for MALDI ToF • 2,5-dihydroxybenzoic acid (DHB) • α-cyano-4-hydroxy-cinnamic acid • 3,5-dimethoxy-4-hydroxycinnamic acid (sinapinic acid) • Specific compounds for glycoprotein etc Schematic of ESI MS developed by John Fenn (taken from Fenn’s Nobel Prize lecture) ETD (Electron Transfer Dissociation) • • • • anion reagent – Fluoranthene fragmentation is superior over CID essential for difficult peptides with PTM enables structural analysis of complex carbohydrate Dual source ESI for LTQ-Orbitrap Thermo LTQ CID ETD Waters Q-ToF MS Waters Q-ToF (Quadrupole time-of-flight mass spectrometer) Quantitative Proteomics • Relative Quantitation • Absolute Quantitation • Typically the answer is the presence or absence of a certain protein • Expression • Interaction • Modifications Top Down Proteomics • 2-D Gel electrophoresis (DIGE) • Mass spec based top down measurement (including using traditional fractionation methods) Middle Down Proteomics • Spear-headed by Neil Kelleher • Uses mild digestion (enzymatic or chemical cleavages) • CNBr – cleaves at methionine • Acetic Acid, Formic Acid - cleaves at aspartic acid • Formylation and acetylation precaution ! Bottom Up Proteomics • • • • • • • • Label Free mass spec method MudPIT Waters High – Low method Stable-isotope labeling method Metabolic ICAT iTRAQ O-18 Absolute Quantitation • Typically using a Triple-Quadrupole MS • Add known amount of specific stable isotope labeled peptide • Also known as Accurate Mass Tagging method (R. Smith) ICAT Applied Biosystems Q-Trap 5500 •hybrid triple quad/ion-trap MS •6 orders of dynamic range •Resolution 3000 Interactome – Cell Research | Vol 18 No 7 | July 2008 – Mapping the human protein interactome – Daniel Figeys - The Ottawa Institute of Systems Biology, The Department of Biochemistry, Microbiology and Immunology, University of Ottawa, – Ottawa, ON, K1H 8M5, Canada – Interactions are the essence of all biomolecules because they cannot fulfill their roles without interacting with other – molecules. Hence, mapping the interactions of biomolecules can be useful for understanding their roles and functions. – Furthermore, the development of molecular based systems biology requires an understanding of the biomolecular – interactions. In recent years, the mapping of protein-protein interactions in different species has been reported, but – few reports have focused on the large-scale mapping of proteinprotein interactions in human. Here, we review the – developments in protein interaction mapping and we discuss issues and strategies for the mapping of the human protein Specific Proteomics Reactor • • • • • Daniel Figeys at Ottawa, Canada Glycomics Reactor Phosphorylation Reactor Ubiquitin Reactor Interactome Reactor Thermo LTQ-FT-ICR-MS •attomole sensitivity •widest dynamic range ( > 4,000) •ppb mass accuracy •resolution >750,000 •ECD Top Down Proteomics • • • • • McLafferty (Cornell) Neil Kelleher (formerly UIUC Chemistry) Uses FT-ICR-MS Middle down approach (mild digestion) Pro-cite program Thermo-LTQ-Hybrid/Orbitrap Thermo-Orbitrap Waters Q-ToF SYNAPT G2 •Newest member of Waters high resolution Q-ToF family of mass spectrometers •First commercial Mass Spectrometer capable of measuring ion-mobility •Uses T-Wave (traveling wave) to improve ion mobility •T-Wave uses RF •Separation by mass, charge and shape Using Ion Mobility MS to differentiate neuropeptides differing by one D/L amino acid • YdAEFL amide • YlAWFL amide • FdMRF amide • FlMRF amide Comparison study between • Thermo LTQ, • Thermo LTQ-Orbitrap • Waters SYNAPT (Slide taken from Bruker Daltonics) Jonathan Sweedler Laboratory (UIUC) Micro-sampling technique Sampling of neuropeptides using SPE beads • Aplysia – abdominal bag cell clusters • PAC (Pleural Abdominal Connective) nerve stimulation • Beads placed before, during PAC stimulation • Neuropeptides varies many orders, verified by MS A A Images obtained from a mouse kidney section. (a) digital photograph (b) total ion count (c) Ion 4,965 Da (d) Ion 11,362 Da MALDI Imaging (taken from Waters literature) Proteomics - Challenges • • • • • • Sample Sampling Handling Complexity Reduction Chromatography Mass Spectrometry Bioinformatics (Abersold (2009) Nature Methods, vol 6, 411) Proteomics Center Carver Biotechnology Center Lab Tours • Wednesday, September 1, 11 AM – 12 Protein Sciences Facility (Noyes Laboratory 315 (SW Corner) • Monday, September 6, Happy LABOR DAY! • Wed, Sept 8, meet in Mumford 11 AM – short lecture – then go to IGB for tour. Questions and Inquiries: Peter Yau (333-3841 [email protected])