* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 3
Endogenous retrovirus wikipedia , lookup
Gene regulatory network wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Metalloprotein wikipedia , lookup
Biochemical cascade wikipedia , lookup
Gene expression wikipedia , lookup
Expression vector wikipedia , lookup
Biochemistry wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Paracrine signalling wikipedia , lookup
Magnesium transporter wikipedia , lookup
Protein structure prediction wikipedia , lookup
Interactome wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Gel electrophoresis wikipedia , lookup
Signal transduction wikipedia , lookup
Protein purification wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Monoclonal antibody wikipedia , lookup
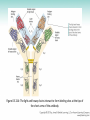
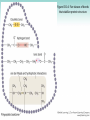
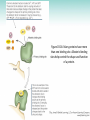
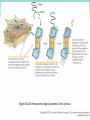
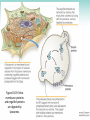
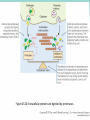
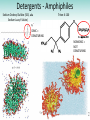
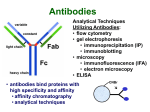
Chapter 3 Proteins and Polypeptides George Plopper Figure 03.13A: The light and heavy chains interact to form binding sites at the tips of the short arms of the antibody. Antibody facts worth knowing • Like all proteins, antibodies can be denatured by SDS and other harsh detergents • Antibodies are proteins secreted by immune cells called B cells. • Antibodies can be isolated from serum (blood) (polyclonal), or from a single B cell colony (monoclonal). • Every different antibody has a unique Fab region. • All antibodies isolated from the same species of animal have nearly identical Fc regions. Figure 03.13B: Each domain in the heavy and light chains is tinted a different color for easier identification. Protein Data Bank ID: 1IGY. Harris, L. J., Skaletsky, E., and McPherson, A., J. Mol. Biol. 275 (1998): 861-872. Figure 03.14: Five classes of bonds that stabilize protein structure. Figure 03.15: Seven different types of molecules can be covalently attached to amino acid side chains. Figure 03.16: Many proteins have more than one binding site. Allosteric binding sites help control the shape and function of a protein. Figure 03.17: Occupancy of an allosteric GTP binding site controls a G protein subunit's shape. Three different conformations of the subunit are overlaid for comparison. Photo courtesy of Heidi E. Hamm and Will Oldham, Vanderbilt University Medical Center. Figure 03.18: Proteasomes digest proteins in the cytosol. Figure 03.19: Most membrane proteins and engulfed proteins are digested by lysosomes. Figure 03.20: Extracellular proteins are digested by proteinases. Methods in Cell & Molecular Biology • Cell fractionation – Detergents – Centrifugation – Gel electrophoresis • Protein isolation and detection – Immunoprecipitation – Western blotting Detergents - Amphiphiles Sodium Dodecyl Sulfate (SDS, aka Sodium Lauryl Sufate) Triton-X-100 IONIC = DENATURING NONIONIC = NOT DENATURING Centrifugation http://www.coleparmer.com/TechLibraryArticle/30 Gel electrophoresis • SDS Polyacrylamide Gel Electrophoresis (SDS PAGE) Western blotting Sample Western Blot Sources • http://biomoleculaire.blogspot.com/ • http://employees.csbsju.edu/hjakubowski/cla sses/ch331/Techniques/TechElectrophoresis.h tm • http://www.labome.com/review/gene/mouse /Irs1-antibody-western-blot.html