* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Dendreon: Pipeline Largely Based on Active Cellular Immunotherapy
Survey
Document related concepts
Immunocontraception wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Immune system wikipedia , lookup
Duffy antigen system wikipedia , lookup
Monoclonal antibody wikipedia , lookup
DNA vaccination wikipedia , lookup
Adaptive immune system wikipedia , lookup
Molecular mimicry wikipedia , lookup
Innate immune system wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Transcript
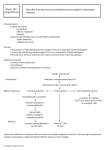
Dendreon: Pipeline Largely Based on Active Cellular Immunotherapy – a Patient-Specific Therapy; Economies of Scale Expected to Allow Commercially Attractive Margins Dendreon’s pipeline is largely based on a core technology that enables activation of a patient’s immune system to attack cancer cells (“active cellular immunotherapy” or cancer vaccine). A key proprietary component is an antigen delivery cassette technology that results in a fusion protein of the cancer-specific antigen (i.e., protein enriched on cancer cells) linked to GM-CSF, an important immune system activating protein. The fusion protein, when combined with harvested immune system cells from a patient, activates the resting antigen presenting cells (APCs, a critical type of cell in the immune system). Once infused into the patient, this final product, which includes the activated APCs, triggers the immune system to attack the cancer cells. This approach results in a >1,000-fold superior immune response than if the patient’s cells were combined with an unmodified antigen. Of note, while this is a patient-specific therapy, economies of scale are expected to allow commercially attractive margins. Products in Dendreon’s pipeline include: • • • • • Provenge. Provenge (sipuleucel-T) is a cancer vaccine for the treatment of metastatic, androgen-independent prostate cancer. Provenge employs the core technology described above and is designed to attack prostate cancer cells that express the prostatic acid phosphatase (PAP) protein. A BLA for Provenge was re-filed in November 2009 and has been assigned a PDUFA date of May 1, 2010. Neuvenge. Neuvenge (lapuleucel-T) is targeting the treatment of cancers expressing the Her2/neu protein. Initiation of a Phase 2 trial of Neuvenge in bladder cancer is expected in 1Q11e. CA-9. CA-9 is expressed in a broad range of tumors, including renal cell, head and neck, cervical and others. A Ph 1/2 RCC trial is planned for 2011. CEA. The company licensed the CEA antigen from Bayer with the aim of applying its platform technology to target the 65% of breast cancers and 70% of lung cancers that express this antigen. A Phase 1 trial is planned for 2012. TRPM8. TRPM8 represents Dendreon’s sole small molecule asset. A Phase 1 trial evaluating D3263, a Trpm8 agonist, is ongoing. Source: JPMorgan/Kasimov, February 22, 2010 Oncology Indication: Prostate Keyword: Clinical Trials/Pipeline