* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download This is the first exam with targeted syntheses that you
Survey
Document related concepts
Enantioselective synthesis wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Bottromycin wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Elias James Corey wikipedia , lookup
Stille reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Aldol reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Petasis reaction wikipedia , lookup
Discodermolide wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Transcript
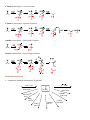
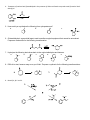
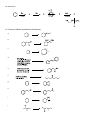
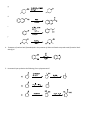
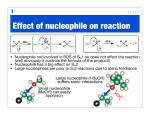
Study Guide for Exam 2- Aldehydes and Ketones Oxidation of Alcohols to Carbonyl Compounds The oxidation of alcohols to carbonyl compounds is the reverse of nucleophilic addition (below). Most oxidants accept the alcohol oxygen as a nucleophile followed by loss of the acidic hydrogen. The process is completed by an E2-like elimination of hydrogen from the proto-carbonyl carbon in concert with formation of the C=O -bond and reductive loss of the leaving group. General Mechanism Swern Chromic Acid Scope and Limitations 1. As a hydrogen atom is needed for the elimination step, 3o alcohols do not oxidize to carbonyl compounds. 2. Normally 1o alcohols are converted to aldehydes and 2o alcohols to ketones. 3. However, in the presence of water, aldehydes form hydrates that undergo more rapid oxidation than the starting 1o alcohols. Thus with CrO3/H2SO4, Na2CrO7, K2CrO7, H2CrO4, 1o alcohols are converted to carboxylic acids. 4. This over-oxidation is avoided with the Swern oxidation or the use of PCC. Nucleophilic Addition Most of the reactions of aldehydes and ketones in these chapters are nucleophilic addition reactions. The oxygen in C=O polarizes the bond. Therefore, while electrophilic addition (electrophile first, followed by nucleophile) was favored for the comparatively non-polar, electron-rich alkene, carbonyls undergo nucleophilic addition (nucleophile first, followed by electrophile). Note how all the mechanisms begin exactly the same way: General Mechanism Hydride LiAlH4 is similar Carbanion Grignard/Alkyllithium/Acetylide Ylide Wittig Reaction The Wittig is unique in that the alkoxide oxygen in the tetrahedral intermediate attacks the phosphonium center forming an oxaphosphetane intermediate. Thus, the electrophile is not H+ as in the previous examples but the phosphonium center. The intermediate undergoes a reverse 2+2 process to form triphenylphosphine oxide and an alkene product to complete the process. If the nucleophile is a weaker base than the alkoxide in the tetrahedral intermediate, an alternative mechanism is proposed. Here, the electrophile (usually H+) is added first to enhance the polarity of the C=O bond, and reduce the energy of the tetrahedral intermediate (transition state resembles this intermediate; stabilizing it will increase the rate). The reverse reaction rates are also enhanced, so the mechanisms feature equillibria. Alcohol as Nucleophile – Acetal/Ketal Formation 1o Amine as Nucleophile – Imine Formation 2o Amine as Nucleophile – Enamine Formation Cyanide as Nucleophile – Cyanohydrin Formation Peracid as Nucleophile – Baeyer-Villager Oxidation Developmental Problems 1. Complete the following ‘reactivity tree’ for a ketone: H 3O+, H 2O ROH, H 3O+ HO H 3O+ PPh 3 O Ph HCN, KCN OH, 1) RMgBr 2) dil. H 3O+ RNH 2, pH 5.5 R 2NH, pH 5.5 NH 2OH, pH 5.5 1) LiAlH 4 2) dil H 3O+ 2. Predict the products: a. b. c. d. e. f. g. h. i. j. 3. Predict the products – Part II: a. b. c. d. e. f. g. h. i. j. 4. Treatment of cathecol with formaldehyde in the presence of dilute acid leads to a product with formula C7H6O2. Identify it! 5. How would you synthesize the following from cyclopentanone? a. b. c. 6. Glutaraldehyde is a germicidal agent used to sanitize surgical equipment that cannot be autoclaved. Propose a mechanism for the following transformation: 7. Hydrolyze the following derivatives back to the original aldehydes and ketones: a. b. c. d. e. 8. Difficult to start; however easy once you finish! Propose a synthesis for the following transformations: 9. Identify A, B, C and D: 10. Identify A-E: 11. Provide an efficient synthesis for the following: a. b. c. d. e. f. g. h. i. j. EXAM PREPARATION Nomenclature: Aldehydes and Ketones Syntheses: This is the first exam with targeted syntheses that you need to work out prior to the exam. You are free to work with your classmates as much as you want (except during the exam of course!). I will not post a key, nor provide you finished syntheses. If you want questions answered you must have made a reaonable attempt (in writing) at solving the synthesis on your own. During review sessions, any student that asks about the syntheses will be asked to go to the board to present what they have worked out so far. I will guide the class towards through any difficulties. Enovid®: This common contraceptive contains the compound norethynodrel. Convert the precursor to this component using any reagents you require. (Hint: at some point you will need to use a protecting group!) OH O OH C CH O Precursor Tamoxifen®: This is a drug used in the treatment of breast cancer. Propose a synthesis of tamoxifen from the following 3’hydroxybenzophenone, benzene and any carbon containing compounds of three carbons or less with any reagents you require. Norethynodrel O OH O N Tamoxifen Ibuprofen (Motrin®, Advil® Nuprin ®) Synthesize this common NSAID (non-steroidal anti-inflammatory drug) from benzene and any other reagents you wish. O C OH Disparlure: This molecule is a sex attractant of the Porthetria dispar gypsy moth. Propose a synthesis of disparlure starting with any two aldehydes and/or ketones you wish as your sole sources of carbon atoms. Assume any Wittig reaction (hint) would give you exclusively the Z-isomer alkene as a product: KEY 1. Complete the following ‘reactivity tree’ for a ketone: HO OH H 3O+, H 2O PPh 3 O Ph Ph ROH, H 3O+ Ph HCN, KCN RO OR Ph HO H 3O+ O OH, 1) RMgBr 2) dil. H 3O+ RNH 2, pH 5.5 O Ph NR R 2NH, pH 5.5 Ph b. c. d. e. f. 1) LiAlH 4 2) dil H 3O+ OH NR 2 Ph 2. Predict the products: a. NH 2OH, pH 5.5 N OH Ph Ph OH Ph OH Ph R CN g. h. i. j. 3. Predict the products – Part II: a. b. c. d. e. f. g. h. i. j. 4. Treatment of cathecol with formaldehyde in the presence of dilute acid leads to a product with formula C7H6O2. Identify it! 5. How would you synthesize the following from cyclopentanone? 6. Glutaraldehyde is a germicidal agent used to sanitize surgical equipment that cannot be autoclaved. Propose a mechanism for the following transformation: 7. Hydrolyze the following derivatives back to the original aldehydes and ketones: 8. Difficult to start; however easy once you finish! Propose a synthesis for the following transformations: 9. Identify A, B, C and D: 10. Identify A-E: 11. Provide an efficient synthesis for the following: a. b. c. d. e. f. g. h. i. j.