* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Aldehydes, Ketones and Carboxylic Acids
Survey
Document related concepts
Cracking (chemistry) wikipedia , lookup
Metal carbonyl wikipedia , lookup
Elias James Corey wikipedia , lookup
Physical organic chemistry wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Carbohydrate wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Aldol reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Transcript
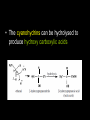
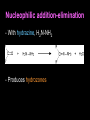
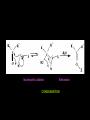
Aldehydes and Ketones AH Chemistry Unit 3(b) Functional Group • What is the functional group of the aldehydes and ketones? • What type of bonds link the C and O atoms? Physical Properties • How do the boiling points compare to alkanes? – Why? • How do the boiling points compare to alcohols? – Why? • Can aldehydes and ketones set up hydrogen bonds with each other? • Can they set them up with water? Chemical reactions • What are the characteristic reactions of aledehydes and ketones? – Reduction – Nucleophilic addition – Nucleophilic addition-elimination • Which is more susceptible to nucleophilic attack, aldehyde or ketone? – Why? Oxidation • What reagents can be used to oxidise aldehydes? Reduction • Aldehydes and ketones can be reduced to produce alcohols. • The reducing agent of choice is LiAlH4 - lithium aluminium hydride • Able to transfer a hydride ion to the partially positive carbon atom of the carbonyl group • Musty be carried out in anhydrous conditions (in ether) Nucleophilic addition • With HCN, producing cyanohydrins.... • The cyanohydrins can be hydrolysed to produce hydroxy carboxylic acids Nucleophilic addition-elimination - With hydrazine, H2N-NH2 - Produces hydrozones Nucleophilic addition Elimination CONDENSATION - With 2,4-dinitrophenylhydrazine - Produces 2,4-dinitrophenylhydrozones These products are “derivatives”. They are crystalline solids with characteristic melting points. The melting point of the derivative can be used to identify the orignial carbonyl compound.