* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Swallowing reflex and brain stem neurons activated by superior
Convolutional neural network wikipedia , lookup
Biological neuron model wikipedia , lookup
Embodied language processing wikipedia , lookup
Haemodynamic response wikipedia , lookup
Adult neurogenesis wikipedia , lookup
Neural engineering wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Aging brain wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Synaptogenesis wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Neuroplasticity wikipedia , lookup
Neuroeconomics wikipedia , lookup
Single-unit recording wikipedia , lookup
Microneurography wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Environmental enrichment wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Axon guidance wikipedia , lookup
Multielectrode array wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Neural oscillation wikipedia , lookup
Mirror neuron wikipedia , lookup
Metastability in the brain wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neural coding wikipedia , lookup
Transcranial direct-current stimulation wikipedia , lookup
Hypothalamus wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Evoked potential wikipedia , lookup
Nervous system network models wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Circumventricular organs wikipedia , lookup
Central pattern generator wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neuroanatomy wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Neurostimulation wikipedia , lookup
Optogenetics wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
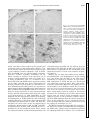
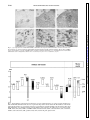
Am J Physiol Gastrointest Liver Physiol 280: G191–G200, 2001. Swallowing reflex and brain stem neurons activated by superior laryngeal nerve stimulation in the mouse Q. SANG AND RAJ K. GOYAL Center for Swallowing and Motility Disorders, West Roxbury Division of Veterans Affairs Boston Healthcare System, West Roxbury 02132; and Harvard Medical School, Boston, Massachusetts 02215 Received 28 February 2000; accepted in final form 24 August 2000 peristalsis is a centrally programmed sequence of oropharyngeal and esophageal contractions that is orchestrated by a so-called swallowing program generator (SPG) in the brain stem (10, 13, 14, 20). The SPG neurons receive input from peripheral oropharyngeal afferents and central cortical projections, which are responsible for activating reflex swallowing (10, 13). The premotor neurons send commands to motoneurons that execute the motor peristaltic sequence. It has been shown that distinct components of the SPG are responsible for oropharyngeal and esophageal phases of swallowing (10, 13, 14). The oropharyngeal swallowing is evoked by oropharyngeal afferents carried primarily in the superior laryngeal nerves (SLN) that project onto the second-order interneurons in interstitial solitary subnuclei (SolI) and intermediate solitary subnuclei (SolIM) (16, 24). Studies (3) using pseudorabies virus as a transneuronal tracer have shown that these subnuclei also contain premotor neurons for motoneurons in semicompact formation of the nucleus ambiguus (NAsc), loose formation of the nucleus ambiguus (NAl), and other cranial nerve nuclei that innervate the oropharyngeal striated muscles. Interneurons in SolI and SolIM also project onto central solitary subnuclei (SolCe) interneurons (8). SolCe neurons are premotor neurons for motoneurons in the compact formation of the nucleus ambiguus (NAc) that innervate the esophageal striated muscle (4, 15). During swallow-evoked primary peristalsis, the oropharyngeal and esophageal components of SPG act in concert. However, the oropharyngeal and esophageal phases may be dissociated (10). Oropharyngeal activity without esophageal peristalsis may occur when SolI and SolIM interneurons fail to activate SolCe interneurons. On the other hand, secondary peristalsis (esophageal peristalsis alone) is elicited when SolCe interneurons are activated by afferents arising from the esophagus (17). The above conclusions apply primarily to the activity in the striated muscle of the oropharynx and esophagus (6, 8, 13, 20). The preganglionic motoneurons for the smooth muscle portion of the esophagus and the lower esophageal sphincter (LES) are located in the dorsal motor nucleus of vagus (DMV) (1, 9, 28, 29, 30); however, location of their premotor neurons is unclear (2, 27). We investigated the swallowing responses elicited by SLN stimulation at various frequencies in mice. Electrical stimulation of the SLN at 10 Hz produced a primary peristalsis, 5 Hz produced oropharyngeal contractions and LES relaxation without esophageal peristalsis, and 1 Hz produced no response. We also examined the location of putative swallow-related neurons in the brain stem that may be involved in these reflex Address for reprint requests and other correspondence: R. K. Goyal, Research and Development (151), VA Boston Health Care System, 1400 VFW Parkway, West Roxbury, MA 02132 (E-mail: [email protected]). The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked ‘‘advertisement’’ in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. c-fos expression; solitary subnuclei; nucleus ambiguus; dorsal motor nucleus of vagus; swallowing program generator SWALLOW-INDUCED PRIMARY http://www.ajpgi.org G191 Downloaded from http://ajpgi.physiology.org/ by 10.220.32.247 on May 12, 2017 Sang, Q., and Raj K. Goyal. Swallowing reflex and brain stem neurons activated by superior laryngeal nerve stimulation in the mouse. Am J Physiol Gastrointest Liver Physiol 280: G191–G200, 2001.— The purpose of the present study was to identify vagal subnuclei that participate in reflex swallowing in response to electrical stimulation of the left superior laryngeal nerve (SLN). SLN stimulation at 10 Hz evoked primary peristalsis, including oropharyngeal and esophageal peristalsis, and LES relaxation. It also induced c-fos expression in interneurons in the interstitial (SolI), intermediate (SolIM), central (SolCe), dorsomedial (SolDM) and commissural (SolC) solitary subnuclei. Neurons in parvicellular reticular nucleus (PCRt) and area postrema (AP) and motoneurons in the semicompact (NAsc), loose (NAl), and compact (NAc) formations of the nucleus ambiguus and both rostral (DMVr) and caudal (DMVc) parts of the dorsal motor nucleus of vagus were also activated. The activated neurons represent all neurons concerned with afferent SLNmediated reflexes, including the swallowing-related neurons. SLN stimulation at 5 Hz elicited oropharyngeal and LES but not esophageal responses and evoked c-fos expression in neurons in SolI, SolIM, SolDM, PCRt, AP, NAsc, NAl, and DMVc but not in SolCe, NAc, or DMVr. These data are consistent with the role of SolI, SolIM, SolDM, NAsc, NAl, and DMVc circuit in oropharyngeal peristalsis and LES relaxation and SolCe, NAc, DMVc, and DMVr in esophageal peristalsis and LES responses. G192 BRAIN STEM NEURONS IN SWALLOWING activities by identifying the c-fos-activated neurons at different SLN stimulus frequencies in separate animals. c-fos is an early response gene that is expressed in transsynaptically activated neurons. Identification of c-fos expression may serve as an important technique to identify neural circuits in reflex activities (11, 22, 26, 34). MATERIAL AND METHODS Downloaded from http://ajpgi.physiology.org/ by 10.220.32.247 on May 12, 2017 Twenty-eight adult male Swiss Webster mice (Taconic, Germantown, NY) weighing 20–40 g were used. The procedures were approved by the Animal Committee of the Brockton/West Roxbury Veterans Affairs Medical Center. Esophageal manometry. Esophageal manometry was performed using a custom-designed catheter assembly (Dentsleeve, Parkside, SA, Australia). The assembly consisted of three silicon catheters (ID, 0.3 mm; OD, 0.6 mm) that were glued together. The catheter was 6.5 cm long and had an outside diameter of 1.2 mm. Each catheter had a 0.3-mm side opening; the side openings were located 2 mm apart. The catheters were filled and continuously perfused with bubble-free preboiled water from a reservoir at a rate of 7 l/min using a pressurized nitrogen chamber. The catheters provided a lowcompliance system and were connected to flow through pressure transducers that were connected to ETH 400 Bridge amplifiers (CB Science, Dover, NH). The output of pressure signals was displayed and recorded on MacLab software (AD Instruments, Castle Hills, NSW, Australia). The mice were anesthetized with a subcutaneous injection of pentobarbital sodium (60 mg/kg) and placed on a heating pad. The catheter assembly was introduced into the esophagus through a gastrostomy. The catheters were then withdrawn gradually in 0.5- to 1-mm increments to scan for the high-pressure zone of the LES. The three catheter openings were positioned in the LES and at 2 and 4 mm above the LES. Esophageal and LES pressure changes in response to a spontaneous swallow and electrical stimulation of the SLN were then recorded. The occurrence of artifactual LES relaxation-associated axial movement of the LES was excluded by careful visual monitoring. Pharyngeal swallowing activity was identified by visually observing the motion of the exposed laryngopharyngeal area in the neck. The pharyngeal pressures were not recorded for technical reasons. A midline neck incision was then made, and the left SLN was exposed. In the mouse, the SLN arises from the vagus below the nodose ganglion and lies dorsal to the carotid artery. The SLN was isolated and severed at the point where it enters the laryngeal musculature. The central end of the SLN was placed on a bipolar (silver-platinum) hook electrode and covered with warm mineral oil and stimulated using a Grass stimulator (model S11; Quincy, MA). Current spread to adjoining tissues caused local muscle contractions and was avoided. The stimulus consisted of square wave pulses of 0.5 ms at 8 V, and stimulation was performed for a period of 9 s at frequencies of 1, 5, or 10 Hz. c-fos expression. The electrical stimuli used to induce c-fos expression were similar to those used for the manometric studies, except that the 9-s stimuli were repeated with a stimulus free interval of 10 s for a period for 30 min. Repeated stimuli with 10-s stimulus-free intervals were applied to avoid decay of the swallowing responses with continuous stimuli (33). At the end of the experiment, the mice were killed with a subcutaneous injection of pentobarbital sodium (100 mg/kg) and perfused transcardially with 60 ml PBS (0.9% NaCl in 0.01 M sodium phosphate buffer, pH 7.0) followed by 60 ml of 4% formaldehyde (4% formaldehyde in 0.1 M sodium phosphate buffer, pH 7.0). The brain stem with attached upper cervical spinal cord was removed and postfixed in the same fixative overnight at 4°C. The fixative was removed with three washes in PBS, and the tissue was stored in PBS containing 1% sodium azide plus 30% sucrose for 24 h at 4°C before being sectioned. Frozen coronal serial sections of 25-m thickness were cut and collected directly on silanecoated slides (Sigma Chemical, St. Louis, MO) so that the orientation of the sections was always the same. The sections were then processed for histochemical and immunohistochemical staining. The first of every three sections was stained with cresyl violet for anatomic landmark studies. The section was washed in water and stained with 1% cresyl violet (Fisher Scientific, Pittsburgh, PA) containing 0.25% acetic acid for 5 min. After being rinsed in water, the section was differentiated in 50% ethanol for 5 min and rinsed again in water. The section was then mounted with the mounting medium (Sigma Chemical). The second section was immunostained for choline acetyltransferase (ChAT) and c-fos. The section was incubated in a mixture of primary antisera that contained a rabbit anti-c-fos (1:3,000, Oncogene Research, Cambridge, MA) and a goat anti-ChAT (1:100, Chemicon International, Temecula, CA) for 16–24 h. The unbound antibodies were removed by three washes in PBS. The section was then incubated in a biotinylated donkey anti-rabbit IgG (1:200, Jackson ImmunoResearch, West Grove, PA) and a biotinylated donkey antisheep IgG (1:500, Jackson ImmunoResearch) for 2 h and Vectastain avidin-biotin complex kit (Vector Laboratory, Burlingame, CA) for another 2 h. Immunoreactivity was revealed using a peroxidase substrate kit (diaminobenzidine, DAB; Vector Laboratory). We distinguished c-fos and ChAT immunoreactivities by the location of the immunoreactive products. c-fos stained the cell nucleus, whereas ChAT stained the cell cytoplasm. ChAT immunoreactivity can be revealed by a donkey anti-sheep IgG even though the primary antibody is a goat anti-ChAT (30). The third section was immunostained for c-fos and neuronal nitric oxide synthase (nNOS). The section was incubated in a mixture of a rabbit anti-c-fos and a rabbit anti-nNOS (1:400, Santa Cruz Biotechnology, Santa Cruz, CA), and the immunoreactivity was revealed by DAB reaction as described above. Again, c-fos and nNOS immunoreactivities were also distinguished by the location of immunoreactive products. c-fos stained the cell nucleus, whereas nNOS stained the cell cytoplasm. All the preparations were examined with a Zeiss Axioplan microscope. Images were captured on a Hamamatsu camera with Openlab software. c-fos-positive neurons per coronal sections that showed the maximum number of activated neurons were quantitated semiquantitatively and scored as follows: ⫺, for none; ⫹, for less than 5; ⫹⫹, for between 5 and 10; ⫹⫹⫹, for between 10 and 50; and ⫹⫹⫹⫹, for greater than 50 c-fos-positive neurons. In the present study, we used posterior limits of area postrema (pAP) as the reference point for describing brain stem levels in craniocaudal orientation. AP neurons are a group of small cresyl violet-stained neurons with a subpopulation of ChAT-positive neurons. The craniocaudal extent of the AP in the mouse is ⬃500 m. Obex was not used as a reference point, because the level of obex is defined differently by different investigators. The solitary subnuclei (Sol) were identified by their relative location to anatomic landmarks, such as the fourth ventricle (IV), AP, solitary tract (SolT), and DMV, and corre- BRAIN STEM NEURONS IN SWALLOWING RESULTS Motor response of esophagus and LES to SLN stimulation. As shown in Fig. 1, during a spontaneous swallow manometric recording showed a propagating pressure wave along the esophageal body and relaxation followed by an aftercontraction of the LES. Pharyngeal swallowing activity that was associated with esophageal manometric response was observed by visual inspection of the exposed pharynx. Electrical stimulation of the central end of the SLN induced esophageal pressure changes in the esophagus and LES with frequency-dependent responses. Electrical stimulation of the SLN at 1 Hz did not induce any obvious pressure changes in either the esophageal body or the LES. Electrical stimulation at 5 Hz induced pharyngeal response and a slight decrease in pressures in the LES, but no esophageal response. SLN stimulation at 10 Hz evoked a pharyngeal as well as an esophageal response, including relaxation followed by an aftercontraction of the LES. Each 9-s stimuli typically evoked two swallowing responses. c-fos expression in vagal subnuclei with SLN stimulation. Successful studies were obtained in 4 or 5 animals in each group receiving SLN stimulation at 1, 5, and 10 Hz. Electrical stimulation of the left SLN induced c-fos expression in neurons that were located over a wide area along the entire rostrocaudal extent of the vagal nuclei. c-fos was expressed bilaterally with activated neurons being more numerous on the ipsilateral than the contralateral side. In the solitary nucleus, c-fos expression was found in several different solitary subnuclei (see Figs. 2, 3, and Fig. 1. Examples of esophageal and lower esophageal sphincter (LES) pressure responses to spontaneous swallowing and superior laryngeal nerve (SLN) stimulation at 1, 5, and 10 Hz. A spontaneous swallow is characterized by an esophageal peristaltic contraction associated with LES relaxation and aftercontraction. SLN stimulation at 5 Hz induced an LES relaxation without esophageal contraction. SLN stimulation at 10 Hz for 9 s elicited 2 episodes of propulsive contractions of the esophageal body and relaxation of the LES followed by an aftercontraction at 10-Hz stimulation. Stimuli were square wave pulses of 0.5 ms. Downloaded from http://ajpgi.physiology.org/ by 10.220.32.247 on May 12, 2017 lated with the cytoarchitectural characteristics as defined by cresyl violet staining and nNOS staining. From a coronal perspective, Sol is broadly divided into a smaller lateral and a larger medial subdivision based on their position in relation to the SolT. The lateral subdivision is further subdivided into four subnuclei: dorsolateral (SolDL), ventrolateral (SolVL), ventral (SolV), and SolI. The medial subdivision is further subdivided into seven subnuclei: SolIM, SolCe, medial (SolM), commissural (SolC), dorsomedial (SolDM), gelatinosus (SolG), and rostrocentral (SolRCe). SolCe can be easily distinguished from the other Sol subnuclei as it stands out as a distinct group of compactly packed nNOS-immunoreactive neurons. SolG is recognized by its acellular gelatinous appearance due to the preponderance of the unmyelinated fibers. In the mouse, parvicellular reticular nucleus (PCRt) is a group of scattered cells that lies just ventral to the Sol and extends further ventrally to NA. In the craniocaudal orientation, PCRt extends ⬃600 m rostrally from the anterior AP (aAP). In the mouse, DMV extended from 1,250 m rostral to and 500 m caudal to the pAP. It was arbitrarily divided into rostral DMV (DMVr) and caudal DMV (DMVc). Immunohistochemical staining with ChAT or nNOS revealed that a subpopulation of DMV neurons were ChAT- or nNOS-immunoreactive neurons. The NA is located in ventral regions of the medulla and in the mouse extends throughout its craniocaudal extent of the medulla. It is easily recognized by the cholinergic nature of its neurons. Similar to that described in the rat (7), in the mouse the dorsal division of NA is composed of three rostrocaudally aligned subdivisions, including NAc, NAsc, and NAl formations, respectively. The ventral division of the NA corresponds to the external formation that extends along the entire length of the medulla. G193 G194 BRAIN STEM NEURONS IN SWALLOWING 7 and Table 1). In SolI, c-fos immunoreactivity was seen with all three stimulus frequencies examined. The number of c-fos-positive neurons increased as the stimulation frequency increased (Fig. 2, A–C, and Table 1). In SolIM, c-fos-positive neurons were observed throughout its rostrocaudal extent, but they were most numerous at a level just rostral to the aAP (500–600 m rostral to pAP). The number of c-fos-positive neurons also increased as the stimulus frequency increased (Fig. 2, D–F, and Table 1). Sparse c-fos-reac- tive neurons were also seen in SolDM and SolCe. In SolCe, c-fos-positive neurons were observed with 10-Hz SLN stimulation but not with 1- or 5-Hz SLN stimulation (Fig. 3). In SolM, few c-fos-positive cells were seen at 1 Hz, and their number did not change with higher stimulus frequencies. We have interpreted these observations as indicating a nonspecific response. In SolC, c-fos-positive neurons were seen with all the stimulus frequencies throughout the rostocaudal extent of SolC. The number progressively increased in Downloaded from http://ajpgi.physiology.org/ by 10.220.32.247 on May 12, 2017 Fig. 2. c-fos expression in the solitary subnuclei (Sol) with SLN stimulation at different frequencies. Coronal sections are shown from different animals processed for c-fos and neuronal nitric oxide synthase (nNOS)immunohistochemical staining. c-fos expression is identified as nuclear staining and nNOS as cytoplasmic staining in neurons. A–C: sections from rostral part of the Sol showing that c-fos-reactive neurons are present in the interstitial Sol (SolI; I) [present lateral to solitary tract (SolT; T)] with SLN stimulation at 1 Hz (A). The c-fos-reactive neurons increased with stimulus frequency of 5 Hz (B) and increased further with 10-Hz stimulation (C). D–F: sections at a level just rostral to the area postrema (AP). In these sections, the central Sol (SolCe; Ce) is easily identifiable as a group of densely packed nNOS-positive neurons. The area between the SolCe and SolT represents the region of the intermediate Sol (SolIM). Note an increase in c-fos-reactive neurons in SolIM with increasing stimulus frequencies. DMV, dorsal motor nucleus of vagus. Some c-fos-positive neurons in the dorsal part of the parvicellular reticular nucleus (PCRt) are shown clearly in E. Note also some c-fos-positive neurons in the dorsomedial Sol (SolDM). Only a rare c-fos-reactive neuron was seen in the medial Sol (SolM), and the number of neurons did not increase with increasing stimulus. IV, fourth ventricle. G–I: sections through caudal part of Sol showing c-fos-positive neurons in commissural subnucleus (SolC; C). There was no obvious increase in c-fospositive neurons with increasing SLN stimulus frequencies in the SolC. cc, Central canal. J–L: sections through mid-AP showing that the number of c-fos-positive neurons increased as the stimulation frequency increased. Scale bars, 100 m. BRAIN STEM NEURONS IN SWALLOWING Table 1. c-fos-Positive neurons in vagal subnuclei with SLN stimulation SolI SolIM SolM SolCe SolDM SolC AP PCRt DMVr DMVc NAsc NAc 1 Hz 5 Hz 10 Hz ⫹ to ⫹⫹ ⫹ ⫺ to ⫹ ⫺ ⫺ ⫹ to ⫹⫹ ⫹⫹ ⫹ ⫺ ⫹ ⫹ ⫺ ⫹⫹ to ⫹⫹⫹ ⫹⫹⫹ ⫺ to ⫹ ⫺ ⫺ to ⫹ ⫹⫹ ⫹⫹⫹ ⫹⫹ to ⫹⫹⫹ ⫺ ⫹ to ⫹⫹ ⫹ to ⫹⫹ ⫺ ⫹⫹⫹ ⫹⫹⫹ ⫺ to ⫹ ⫹ ⫹ ⫹⫹ ⫹⫹⫹⫹ ⫹⫹ to ⫹⫹⫹ ⫹ ⫹ to ⫹⫹ ⫹ to ⫹⫹ ⫹ the caudal part of the subnucleus (at levels of 700–850 m). However, the number of active neurons did not change with increasing stimulus frequencies, suggesting that these neurons may not be related to swallowing function. In addition to SolI, SolIM, SolDM, SolCe, and SolC, SLN stimulation also evoked c-fos in the ventrolateral solitary subnucleus (SolVL), which is concerned with respiratory functions. Other solitary subnuclei including SolDL, SolV, SolRCe, SolM, and SolG did not show any c-fos expression with SLN stimulation. SLN stimulation also evoked c-fos expression in the PCRt ,which lies just ventral to the Sol (Fig. 2E). The c-fos was expressed bilaterally with ipsilateral predominance. SLN stimulation also induced c-fos in neurons in the AP (Fig. 2, J–L). The active neurons were distributed throughout the core of the AP and not restricted to the rim of the AP as seen with the stimulation of subdiaphragmatic vagal afferents (29). As in the premotor neurons in solitary subnuclei, c-fos expression also showed a frequency-dependent pattern in the vagal motor subnuclei. SLN stimulation evoked c-fos in NAc and rostral part of NAsc. In the NAsc, c-fos-positive neurons were seen with all the frequencies examined (Fig. 4, A–C). However, in the NAc no c-fos-positive neurons were found with 1- or 5-Hz stimulation but some c-fos-positive neurons were found with 10-Hz stimulation (Fig. 4, D and E). In the DMV, the pattern of c-fos-positive neurons in response to SLN stimulation was different in the rostral and caudal parts. In the DMVr, c-fos-positive neurons were seen with 10-Hz stimulation but not with 1or 5-Hz stimulation (Fig. 5). Even with 10-Hz stimulation, only a small number of DMVr neurons showed c-fos immunoreactivity. In contrast, in the DMVc, c-fospositive neurons were found with all three frequencies of SLN stimulation (Fig. 6). The number of neurons expressing c-fos with SLN stimulation is summarized in Table 1. The c-fos expres- Fig. 3. c-fos expression in the SolCe with 5- and 10-Hz SLN stimulation. Coronal sections were processed for cfos (nuclear) and nNOS (cytoplasmic) double immunostaining. In A (low power) and A⬘ (high power), lack of c-fosimmunoreactive neurons is shown in the SolCe with 5-Hz stimulation. In B (low power) and B⬘ (high power) 1 c-fos-positive neuron (arrow) is shown in the SolCe with 10-Hz stimulation. A and B: scale bars, 100 m. A⬘ and B⬘: scale bars, 50 m. Downloaded from http://ajpgi.physiology.org/ by 10.220.32.247 on May 12, 2017 ⫺, Not detected; ⫹, the highest number of positive cells on a single section was ⬍5; ⫹⫹, the highest number of positive cells on a single section was between 5 and 10; ⫹⫹⫹, the highest number of positive cells on a single section was between 10 and 50; ⫹⫹⫹⫹, the highest number of positive cells on a single section was ⬎50. SLN, superior laryngeal nerve; Sol, solitary subnucleus; SolI, interstitial Sol; SolIM, intermediate Sol; SolM, medial Sol; SolCe, central Sol; SolDM, dorsomedial Sol; SolC, commissural Sol; AP, area postrema; PCRt, parvicellular reticular nucleus; DMV, dorsal motor nucleus of vagus. DMVr, rostral DMV; DMVc, caudal DMV; NA, nucleus ambiguus; NAsc, semicompact formation of NA; NAc, compact formation of NA. G195 G196 BRAIN STEM NEURONS IN SWALLOWING sion of vagal subnuclei in their craniocaudal extent with 10-Hz SLN stimulation is summarized in Fig. 7. DISCUSSION The brain stem circuit involved in swallowing has been investigated using a variety of techniques (2, 5, 6, 14). These include studies (24, 31) of swallowing responses after microlesions or microstimulation of welldefined medullary regions, electrical activity in the identified neurons in response to afferent stimuli that elicit reflex swallowing, and morphological tracer studies (2–4, 9, 18, 19) that show connectivity of neurons in the circuit. Together, they have produced important information on the neural circuit concerned with reflex swallowing. The above studies have been carried out in a number of different animal species. Although no studies have been reported in the mouse, available studies show that despite some obvious species differences, the general organization of the swallowing circuit across animal species is fairly similar. The present studies using esophageal manometry and c-fos expression in brain stem nuclei provide information on functional anatomy of the medullary neurons that may constitute the swallowing-related neurons in the mouse. Moreover, by using the different stimulus parameter of the SLN to elicit different esophageal and LES responses, these studies also help identify the medullary neurons that may be involved in different components of swallowing responses. Although identification of the brain stem swallowing circuit using c-fos expression as a marker has not been described before, this technique has been used widely to identify neurons involved in a variety of reflexes (11, 22, 26, 34). The results of c-fos expression studies require careful interpretation and correlation with results of studies using different techniques. This is because c-fos expression may not directly correlate with a reflex response. For example, c-fos is not expressed in neurons with inhibitory links to SLN input. Even in neurons with an excitatory link to SLN input, c-fos expression is most marked in the first-order neurons, and it decreases progressively in second- and third-order neurons. Stimulation of the SLN afferents elicits a variety of swallow-related and other reflexes, including gagging, retching, coughing, laryngeal spasm, bronchoconstriction, and apnea. These reflexes use overlapping neural circuits. However, the type of reflex evoked by the SLN stimulation depends on the stimulus parameters used (5, 10, 25). In the mouse, SLN stimulation at 10 Hz elicited a full swallowing response, consisting of oropharyngeal and esophageal components including LES relaxation. Stimulation at 5 Hz produced only oropharyngeal and LES relaxation without evoking an esophageal response. Much stronger SLN stimulation (⬎10 Hz) produced apnea. Therefore we did not use stimuli with frequencies ⬎10 Hz to avoid activation of other SLN-mediated reflexes. Even so, it is possible that some of the c-fos-expressing neurons observed in this study may be concerned with functions other than reflex swallowing. Although fiber tracing or electrophysiological studies dealing with reflex swallowing are not available in the Downloaded from http://ajpgi.physiology.org/ by 10.220.32.247 on May 12, 2017 Fig. 4. c-fos expression in nucleus ambiguus (NA) with SLN stimulation. Coronal sections processed for c-fos (nuclear) and choline acetyltransferase (ChAT) (cytoplasmic) double immunostaining. The NA can be recognized by the presence of a group of ChAT-immunoreactive neurons. A–C: c-fos-positive neurons (arrows) in the semicompact formation of the NA (NAsc) with stimuli at 1, 5, and 10 Hz in 3 different mice. D and E: c-fos reactivity in compact formation of NA (NAc) with stimuli at 5 and 10 Hz in 2 different mice. Note the absence of nuclear c-fos expression with 5 Hz (D) and the presence of c-fos-positive neurons (arrow) with a 10-Hz stimulus (E). Cytoplasmic ChAT staining in neurons is evident in both D and E. Scale bars, 50 m. BRAIN STEM NEURONS IN SWALLOWING G197 mouse, such data in other animal species provide good correlation with c-fos data in the mouse. Studies (5, 12, 18, 19, 24) have shown that SLN afferents project onto a large number of solitary subnuclei, such as SolVL, SolI, and SolIM, that are concerned with respiratory protective reflexes, including swallowing. In the mouse, neurons in all these areas expressed c-fos in response to SLN stimulation. In the rat, studies (2, 3, 8) using pseudorabies virus as a neurotracer have shown that SolI and SolIM contain premotor neurons for the NAsc and NAl that house motoneurons for pharyngeal muscles. In the mouse, we found that electrical stimulation of the SLN evoked c-fos expression in interneurons in SolI and SolIM and in motoneurons in NAsc and NAl. SolI and SolIM neurons have also been shown to project onto SolCe (8) that contain premotor neurons for NAc motoneurons innervating the esophageal striated muscle (4). SLN stimulation at 5 Hz produced only the oropharyngeal phase of swallowing, and stimulation at 10 Hz produced a full swallowing response. Therefore, in the mouse, interneurons in SolI and SolIM and motoneurons in NAsc and NAl may constitute the brain stem circuit for oropharyngeal swallowing. Interneurons in SolI, Sol IM, and SolCe and motoneurons in NAsc, NAl, and NAc may be responsible for the full swallowing reflex. These results are consistent with the results of microstimulation and electrophysiological studies (10, 16) showing that the dorsal part of the SPG that includes the region of the SolT nucleus is responsible for the coordination of the oropharyngeal phase as well as the complete swallowing sequence. Studies (4, 6, 17) using extracellular unit recordings, microstimulation, and morphological circuit tracing have also shown that the SolCe is the main site of termination of esophageal afferents. In our studies, SLN stimulation at 10 Hz but not at 5 Hz showed c-fos-expressing neurons in SolCe, NAc, and esophageal body contractions. Moreover, the number of activated neurons even at 10 Hz was small. The reason for this low level of activated neurons is not clear; however, it may be related at least in part to the fact that SolCe interneurons represent second- or third-order neurons in the swallowing circuit. These observations suggest that the neural circuit responsible for the esophageal phase of the peristalsis requires higherintensity SLN simulation than that for the pharyngeal phase of the peristalsis and are consistent with the view that SolCe-NAc may form the brain stem circuit for esophageal peristalsis (4, 8, 17). The SPG was originally mapped into two regions (13, 14). The dorsal region is constituted by Sol and adjoining PCRt and a ventral region encompassing NA and the adjoining PCRt. These studies (13, 14) and others Downloaded from http://ajpgi.physiology.org/ by 10.220.32.247 on May 12, 2017 Fig. 5. c-fos expression in rostral DMV (DMVr) with 5- and 10-Hz SLN stimulation. Coronal sections processed for c-fos (nuclear staining) and ChAT (cytoplasm staining) double immunostaining. The location of DMVr can be recognized as a group of ChAT-positive neurons. In A (low power) and A⬘ (high power), a lack of c-fos-immunoreactive neurons is shown in DMVr with 5-Hz stimulation. In B (low power) and B⬘ (high power), a c-fos-positive neuron is shown (arrow) in the DMVr with 10-Hz stimulation. A and B: scale bars, 100 m. A⬘ and B⬘: scale bars, 50 m. G198 BRAIN STEM NEURONS IN SWALLOWING Fig. 7. Diagrammatic representation of subnuclei of vagal complex showing c-fos-active neurons (shaded area) with 10-Hz SLN stimulation. The level of 0 represents the posterior border of the AP. The darkness of the shade represents the density of c-fos-positive neurons in vagal subnuclei, with the darkest being the densest. Note that SLN stimulation at 10 Hz that evoked a full swallowing motor response also induced c-fos in neurons in SolI, SolIM, SolCe, NAc, NAsc, DMVc, and DMVr. SolDL, dorsolateral Sol; SolVL, ventrolateral Sol; SolV, ventral Sol; SolRCe, rostrocentral Sol; SolG, gelatinous Sol; aAP, anterior AP; pAP, posterior AP. Downloaded from http://ajpgi.physiology.org/ by 10.220.32.247 on May 12, 2017 Fig. 6. c-fos expression in caudal DMV (DMVc) with different frequency SLN stimulation. Coronal sections were processed for c-fos (nuclear staining) and ChAT (cytoplasm staining) double immunostaining. The location of DMVc can be recognized as a group of ChAT-positive neurons. Neurons expressing c-fos (arrowheads) were found with stimulation at all 3 frequencies shown. A–C: scale bars, 100 m. A⬘–C⬘: scale bars, 50 m. BRAIN STEM NEURONS IN SWALLOWING these neurons without producing a motor response. It has been shown that SLN stimulation that does not evoke swallowing activity can evoke electrical activity in the swallowing neurons (16). The frequency-dependent motor responses and c-fos expression in response to SLN stimulation further support the validity of the results with SLN stimulation. In summary, these studies show that SLN stimulation evokes swallowing and peristalsis and c-fos expression in interneurons constituting the putative SPG and motoneurons in the brain stem. These findings are consistent with the view that SolI, SolIM, NAsc, and NAl neurons are responsible for oropharyngeal swallowing and that SolCe and NAc neurons form the circuit for esophageal striated muscle peristalsis. Both these circuits are involved in primary peristalsis. Further studies are needed to more precisely determine the roles of PCRt in reflex swallowing and define links that are responsible for bilateral integration of the two hemi SPGs. Studies are also needed to test whether SolDM, rather than SolCe or SolM, house premotor neurons for the DMVc neurons that mediate LES relaxation during reflex swallowing. We thank Drs. Priyattam Shiromani and D. V. Sivarao for technical advice and Donna Kantarges for editorial assistance. This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Research Grant DK-31092 and a Merit Review Award from the Office of Research and Development, Medical Research Service, Department of Veterans Affairs. REFERENCES 1. Abrahams TP and Hornby PJ. Lower esophageal sphincter relaxation evoked by stimulation of the dorsal motor nucleus of the vagus in ferrets (Abstract). Gastroenterology 116: A589, 1999. 2. Altschuler SM, Bao X, Bieger D, Hopkins DA, and Miselis RR. Viscerotopic representation of the upper alimentary tracts in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tract. J Comp Neurol 283: 248–268, 1989. 3. Bao X, Wiedner EB, and Altschuler SM. Transsynaptic localization of pharyngeal premotor neurons in rat. Brain Res 696: 246–249, 1995. 4. Barrett RT, Bao X, Miselis RR, and Altschuler SM. Brain stem localization of rodent esophageal premotor neurons revealed by transneuronal passage of pseudorabies virus. Gastroenterology 107: 728–737, 1994. 5. Bellingham MC and Lipski J. Morphology and electrophysiology of superior laryngeal nerve afferents and postsynaptic neurons in the medulla oblongata of the cat. Neuroscience 48: 205–216, 1992. 6. Bieger D. Central nervous system control mechanisms of swallowing: a neuropharmacological perspective. Dysphagia 8: 308– 310, 1993. 7. Bieger D and Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol 262: 546–562, 1987. 8. Broussard DL, Lynn RB, Wiedner EB, and Altschuler SM. Solitarial premotor neuron projections to the rat esophagus and pharynx: implications for control of swallowing. Gastroenterology 114: 1268–1275, 1998. 9. Collman PI, Tremblay L, and Diamant NE. The central vagal efferent supply to the esophagus and lower esophageal sphincter of the cat. Gastroenterology 104: 1430–1438, 1993. 10. Doty RW, Richmond WH, and Storey AT. Effect of medullary lesions on coordination of deglutition. Exp Neurol 17: 91–106, 1967. 11. Fields RD, Eshete F, Stevens B, and Itoh K. Action potentialdependent regulation of gene expression: temporal specificity in Downloaded from http://ajpgi.physiology.org/ by 10.220.32.247 on May 12, 2017 using microelectrode recordings and chemostimulation techniques have suggested that PCRt may play an important role in reflex swallowing. In the present study, we found that SLN stimulation evoked c-fos expression in PCRt neurons, which is consistent with the view of a possible role for PCRt neurons in reflex swallowing (13, 14, 31, 32). The LES in all animal species, including the mouse, and the distal esophagus in many species are composed of smooth muscles that receive preganglionic motor innervation from DMV motoneurons (1, 9, 27–29). Several studies (1, 28) have suggested that DMVr and DMVc neurons provide excitatory and inhibitory inputs, respectively, to the smooth muscle of the distal esophagus and LES. In the present study, 10-Hz SLN stimulation elicited a full swallowing response, including LES relaxation and aftercontraction, and induced c-fos expression in both the DMVr and DMVc. On the other hand, 5-Hz SLN stimulation elicited isolated LES relaxation only and induced c-fos expression solely in DMVc neurons. These observations are consistent with those of previous studies (1, 21, 25, 27, 28) in other species finding that inhibitory responses of the LES are more sensitive to afferent stimulation at intensities lower than the excitatory responses. Dendrites of DMV motoneurons have been shown to penetrate and make contacts with neurons in SolM and SolDM but not in SolCe (2, 23). In the present study, SLN stimulation at 5 Hz activated neurons in SolDM and DMVc but not in SolM or DMVr. Therefore it is possible that SolDM may contain premotor neurons for DMVc motoneurons that are involved in swallowevoked LES relaxation. Recent studies (27) have shown that SolCe neurons project onto DMVc as well as DMVr motoneurons, suggesting that the SolCe-DMVc/DMVr neural circuit may also regulate LES function in primary peristalsis. Further studies are needed to resolve this important issue. Studies in several animal species have shown that SLN afferents project onto SolI, SolIM, and SolCe neurons only ipsilaterally. However, ipsilateral SLN stimulation activates these nuclei bilaterally. The neural connections responsible for bilateral integration of swallowing with ipsilateral SLN stimulation are not known. Studies (5, 18, 19, 31) in the cat have shown that afferent fibers of ipsilateral SLN project bilaterally onto SolC over a 2.5-mm area. However, functional studies (33) do not reveal a role for SolC in swallowing. Therefore, c-fos activity in SolC may not be related to swallowing. Recent studies (31) in the cat have suggested that the bilateral integration of swallowing activity may occur at the level of PCRt that receives afferent projections from SLN bilaterally and make contralateral connections between the half SPGs on either side of the midline. Low-frequency (1 Hz) SLN stimulation evoked no oropharyngeal or esophageal motor activity. However, it induced c-fos in a few neurons in some of the subnuclei. c-fos expression has been shown to correlate with electrical spike potentials in neurons (26). Therefore, it is possible that SLN stimulation at 1 Hz activates G199 G200 12. 13. 14. 15. 16. 17. 19. 20. 21. 22. 23. Ca2⫹, cAMP-responsive element binding proteins, and mitogenactivated protein kinase signalling. J Neurosci 17: 7252–7266, 1997. Hashim MA and Bieger D. Excitatory amino acid receptormediated activation of solitarial deglutitive loci. Neuropharmacology 28: 913–921, 1989. Jean A. Brainstem organization of the swallowing network. Brain Behav Evol 25: 109–116, 1984. Kessler JP and Jean A. Identification of the medullary swallowing regions in the rat. Exp Brain Res 57: 256–263, 1985. Kobler JB, Datta S, Goyal. RK, and Benecchi EJ. Innervation of the larynx, pharynx, and upper esophageal sphincter of the rat. J Comp Neurol 349: 129–147, 1994. Lang IM, Medda BK, Ren J, and Shaker R. Characterization and mechanisms of the pharyngoesophageal inhibitory reflex. Am J Physiol Gastrointest Liver Physiol 275: G1127–G1136, 1998. Lu WY and Bieger D. Vagal afferent transmission in the NTS mediating reflex responses of the rat esophagus. Am J Physiol Regulatory Integrative Comp Physiol 274: R1436–R1445, 1998. Lucier GE, Egizii R, and Dostrovsky JO. Projections of the internal branch of the superior laryngeal nerve of the cat. Brain Res Bull 16: 713–721, 1986. Mifflin SW. Laryngeal afferent inputs to the nucleus of the solitary tract. Am J Physiol Regulatory Integrative Comp Physiol 265: R269–R276, 1993. Miller AJ. The search for the central swallowing pathway: the quest for clarity. Dysphagia 8: 185–194, 1993. Mittal RK, Holloway RH, Penagini R, Blackshaw LA, and Dent J. Transient lower esophageal sphincter relaxation. Gastroenterology 109: 601–610, 1995. Morgan JI and Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci 14: 421–451, 1991. Norgren R and Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol. 207–223, 1988. 24. Ootani S, Umezaki T, Shin T, and Murata Y. Convergence of afferents from the SLN and GPN in cat medullary swallowing neurons. Brain Res Bull 37: 397–404, 1995. 25. Paterson WG, Rattan S, and Goyal RK. Experimental induction of isolated lower esophageal sphincter relaxation in anesthetized opossums. J Clin Invest 77: 1187–1193, 1986. 26. Rinaman L, Verbalis JG, Stricker EM, and Hoffman GE. Distribution and neurochemical phenotypes of caudal medullary neurons activated to express c-fos following peripheral administration of cholecystokinin. J Comp Neurol 338: 475–490, 1993. 27. Rogers RC, Hermann GE, and Travagli RA. Brainstem pathways responsible for esophageal control of gastric motility and tone in the rat. J Physiol (Lond) 514: 369–383, 1999. 28. Rossiter CD, Norman WP, Jain M, Hornby PJ, Benjamin S, and Gillis RA. Control of lower esophageal sphincter pressure by two sites in dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol 259: G899–G906, 1990. 29. Sang Q and Goyal RK. Lower esophageal sphincter relaxation and activation of medullary neurons by subdiaphragmatic vagal stimulation in the mouse. Gastroenterology. In press. 30. Sang Q and Young HM. The origin and development of the vagal and spinal innervation of the external muscle of the mouse esophagus. Brain Res 809: 253–268, 1998. 31. Sugimoto T, Umezaki T, Narikawa K, and Shin T. Crossing inputs of the superior laryngeal nerve afferents to medullary swallowing-related neurons in the cat. Neurosci Res 30: 235– 245, 1998. 32. Umezaki T, Matsuse T, and Shin T. Medullary swallowingrelated neurons in the anesthetized cat. Neuroreport 9: 1793– 1798, 1998. 33. Weerasuriya A, Bieger D, Hockman CH. Interaction between primary afferent nerves in the elicitation of reflex swallowing. Am J Physiol Regulatory Integrative Comp Physiol 239: R407– R414, 1980. 34. Willing AE, and Berthoud H-R. Gastric distension-induced c-fos expression in catecholaminergic neurons of rat dorsal vagal complex. Am J Physiol Regulatory Integrative Comp Physiol 272: R59–R67, 1997. Downloaded from http://ajpgi.physiology.org/ by 10.220.32.247 on May 12, 2017 18. BRAIN STEM NEURONS IN SWALLOWING