* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Szerkezetvizsgálati módszerek a biofizikában_2016_opt_mikr_1
Non-coding RNA wikipedia , lookup
Gene expression profiling wikipedia , lookup
Bottromycin wikipedia , lookup
Genome evolution wikipedia , lookup
RNA polymerase II holoenzyme wikipedia , lookup
Eukaryotic transcription wikipedia , lookup
Non-coding DNA wikipedia , lookup
Molecular cloning wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Gene desert wikipedia , lookup
List of types of proteins wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Gene therapy wikipedia , lookup
Gene expression wikipedia , lookup
Point mutation wikipedia , lookup
Molecular evolution wikipedia , lookup
Genomic library wikipedia , lookup
Genetic engineering wikipedia , lookup
Gene regulatory network wikipedia , lookup
Transformation (genetics) wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Expression vector wikipedia , lookup
Community fingerprinting wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Silencer (genetics) wikipedia , lookup
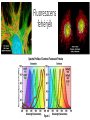
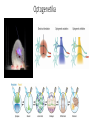
Optikai mikroszkópok I. Konjugált síkok a modern mikroszkópokban. Inverz, fáziskontraszt, epifluoreszcens mikroszkópok. Fluoreszcens fehérjék és optogenetika. Irodalom: www.microscopyu.com Modern mikroszkópok felépítése, végtelenre korrigált optika Konjugált síkok Fáziskontraszt mikroszkóp Fáziskontraszt mikroszkóp Epifluoreszcens mikroszkóp Fluoreszcens fehérjék A GFP szerkezete EYFP vektor The basic plasmid vector configuration useful in fluorescent protein gene transfer experiments has several requisite components. The plasmid must contain prokaryotic nucleotide sequences coding for a bacterial replication origin for DNA and an antibiotic resistance gene. These elements, often termed shuttle sequences, allow propagation and selection of the plasmid within a bacterial host to generate sufficient quantities of the vector for mammalian transfections. In addition, the plasmid must contain one or more eukaryotic genetic elements that control the initiation of messenger RNA transcription, a mammalian polyadenylation signal, an intron (optional), and a gene for co-selection in mammalian cells. Transcription elements are necessary for the mammalian host to express the gene fusion product of interest, and the selection gene is usually an antibiotic that bestows resistance to cells containing the plasmid. These general features vary according to plasmid design, and many vectors have a wide spectrum of additional components suited for particular applications. Brainbow Optogenetika Demonstration of single-component optogenetic control of neurons with microbial opsins4 was followed by corresponding optogenetic terminology2 in October 2006, and corresponding optogenetic control of freely moving mammals using microbial opsins and the fiberoptic neural interface9, 10. Also marked are identifications of bacteriorhodopsin3, halorhodopsin5 and channelrhodopsin6, all of which were much later (2005–2010) shown to function as fast, singlecomponent optogenetic tools in neurons. Numbers indicate only publications searchable by 'optogenetics' or derivatives thereof on 1 December 2010. Optogenetika