* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Comment in GO: This term is intended to
Survey
Document related concepts
Metalloprotein wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Magnesium transporter wikipedia , lookup
Expression vector wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Interactome wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Western blot wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein purification wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Transcript
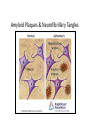
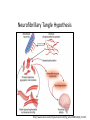
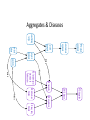
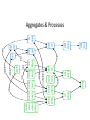
Neurofibrillary Tangles and Amyloid Plaques: The Treatment of Protein Aggregates in the Protein Ontology Alexander P. Cox and Alexander D. Diehl University at Buffalo (SUNY) Protein Ontology Workshop Georgetown University June 18-19, 2014 Existing Terminology in GO & PRO macromolecular complex (GO:0032991) – A stable assembly of two or more macromolecules, i.e. proteins, nucleic acids, carbohydrates or lipids, in which the constituent parts function together. protein complex (GO:0043234) – Any macromolecular complex composed of two or more polypeptide subunits, which may or may not be identical. Protein complexes may have other associated non-protein prosthetic groups, such as nucleotides, metal ions or carbohydrate groups. Comment in GO: This term is intended to exclude structures composed of the same repeating subunit or subunits, for example microtubules. Protein complexes encompassed by this term are generally not structural, and usually have a defined set of subunits. Existing Terminology in GO & PRO macromolecular complex (GO:0032991) – A stable assembly of two or more macromolecules, i.e. proteins, nucleic acids, carbohydrates or lipids, in which the constituent parts function together. protein complex (GO:0043234) – Any macromolecular complex composed of two or more polypeptide subunits, which may or may not be identical. Protein complexes may have other associated non-protein prosthetic groups, such as nucleotides, metal ions or carbohydrate groups. Comment in GO: This term is intended to exclude structures composed of the same repeating subunit or subunits, for example microtubules. Protein complexes encompassed by this term are generally not structural, and usually have a defined set of subunits. What is a Protein Aggregate? Common Conceptions – Necessarily composed of misfolded proteins – Necessarily disorganized – Necessarily non-soluble – Necessarily pathological or indicative of pathology What is a Protein Aggregate? Common Misconceptions – Necessarily composed of misfolded proteins • Not always – immune complexes are protein aggregates – Necessarily disorganized • Not always – prions polymerize in a regular, efficient way – Necessarily non-soluble • Not always – solubility is a secondary characteristic of protein aggregates that depends on size, hydrophobicity, etc. – Necessarily pathological or indicative of pathology • Not always – immune complexes and the formation of fibrin aggregates in coagulation are normal physiological responses Representing Protein Aggregates BFO:object aggregate macromolecule aggregate protein aggregate amyloid plaque has part has part has part ChEBI:macromolecule PR:amino acid chain PR:amyloid beta 42 peptide Macromolecule Aggregate Working definition: An object aggregate whose primary constituents are two or more macromolecules assembled in varying configurations from instance to instance. A macromolecule aggregate may contain secondary constituents such as small molecules or ions. – Elucidation 1: A primary constituent is a constituent that compromises the majority of the mass of the aggregate. – Elucidation 2: A varying configuration includes the possibility of varying the amount of the primary constituent as well as the way the primary constituent is assembled within an instance of a macromolecule aggregate. Protein Aggregate Working definition: A macromolecule aggregate whose primary constituents are proteins assembled in varying configurations from instance to instance. – Elucidation 1: Proteins compromise the majority of the mass of the aggregate. – Elucidation 2: A varying configuration includes the possibility of varying the amount of the individual proteins as well as the way these proteins are assembled within an instance of a protein aggregate. Types of Protein Aggregates – Semi-disordered aggregations of normally folded proteins – Disordered aggregations of misfolded proteins – Protein polymers or oligomers assembled in a regular structure – Fibril aggregates Types of Protein Aggregates – Semi-disordered aggregations of normally folded proteins • immune complexes • activated proteins of the clotting cascade • aggregates of foreign protein, typically left after virus replication – Disordered aggregations of misfolded proteins • misfolded proteins in endoplasmic reticulum or cytoplasm • some inclusion bodies (e.g. IPODs, JUNQs, aggresomes) – Protein polymers or oligomers assembled in a regular structure • microtubules • polymerized prions • myosin fibers – Fibril aggregates • amyloids (e.g. amyloid plaques, Lewy bodies) • neurofibrillary tangles Immune Complexes Proposed definition: An immune complex is a protein aggregate comprised of a network of immunoglobulins bound to epitopes on cognate antigens. An immune complex may include immunoglubulins of varying affinity and isotype. Image From: http://www2.estrellamountain.edu/faculty/farabee/biobk/biobookimmun.html Aggregates of Misfolded Proteins http://cardiovascres.oxfordjournals.org/content/85/2/253/F1.expansion IPODs & JUNQs Wikipedia: IPOD and JUNQ Prions Wikipedia: Prion Amyloids – Insoluble fibrous protein aggregates with a beta sheet structure – Typically extracellular – Can be composed of various proteins (e.g. amyloid beta, alphasynuclein, huntingtin, ubiquitin) and typically contain serum amyloid P-component – Typically, though not always, associated with pathology • melanosomes are nonpathological amyloids composed of the Pmel17 protein Diseases Featuring Amyloids Wikipedia: Amyloid Protein Aggregates & Associated Diseases Aggregate Disease amyloid amyloidosis amyloid plaque Alzheimers disease neurofibrillary tangle Alzheimers disease Lewy body Parkinsons disease; Lewy body dementia polymerized prions prion disease (e.g. Creutzfeldt-Jacob disease) Rosenthal fiber Alexander disease Mallory body Wilsons disease; alcoholic liver disease intraneuronal ubiquitin inclusion motor neuron disease intranuclear Huntingtin inclusion Huntingtons disease Bunina body amyotropic lateral sclerosis Pick body Pick’s disease glial cytoplasmic inclusion multiple system atrophy misfolded beta-hexosaminidase Tay-Sachs disease straight filaments progressive supranuclear palsy The Neurological Disease Ontology (ND) – Extends the Ontology for General Medical Science (OGMS) to represent diseases that affect the human nervous system – Contains approximately 200 subclasses of ‘neurological disease’ classified based on the primary disease mechanism – Provides a comprehensive representation of neurological diseases that includes the disease, disease course, syndrome, disorder, signs and symptoms, diagnosis, diagnostic guideline, and material/genetic basis When is Protein Aggregation Pathological? In general, a biological process can be pathological because: – Whenever present, it is always leads to a disorder – It occurs too frequently – It occurs too infrequently – Alternatively, process A can be said to become pathological when another process B slows down or stops such that the effects of process A then becomes toxic • For example, amyloid aggregation may become pathological only when amyloid aggregate clearance stops working properly and allows the buildup of dangerous levels of amyloid plaques. Tangles, Plaques, & Lewy Bodies Neurofibrillary tangles – Composed primarily of hyperphosphorylated tau – Associated with Alzheimer’s disease and frontotemporal dementia Amyloid plaques – Composed primarily of amyloid beta – Associated with Alzheimer’s disease Lewy bodies – Composed primarily of alpha-synuclein and ubiquitin – Associated with Lewy body disease and Parkinson’s disease Aggregates BFO: object aggregate protein aggregate Lewy body amyloid aggregate macromolecule aggregate neurofibrillary tangle amyloid plaque Amyloid Plaques & Neurofibrillary Tangles Amyloid Plaques & Neurofibrillary Tangles http://www.nature.com/nrn/journal/v9/n7/box/nrn2420_BX1.html Representing Causal Hypotheses in ND • testable hypothesis (OBI_0001908) – An information content entity that expresses an assertion that is intended to be tested. • Alzheimers disease neurofibrillary tangle hypothesis – The testable hypothesis that the degree of dementia resulting from Alzheimers disease more directly correlates with the formation of neurofibrillary tangles than amyloid plaques in the brain. • Alzheimers disease amyloid cascade hypothesis – The testable hypothesis that Alzheimers disease is caused, at least in part, by the accumulation of amyloid plaques in the cortex, hippocampus, basal forebrain, or brain stem regions of the brain. Neurofibrillary Tangle Hypothesis http://www.nature.com/nm/journal/v11/n8/fig_tab/nm0805-826_F1.html Amyloid Cascade Hypothesis Biochemical Society Transactions (2005) 33, 553-558 - www.biochemsoctrans.org Aggregates BFO: object aggregate protein aggregate Lewy body amyloid aggregate macromolecule aggregate neurofibrillary tangle amyloid plaque Proteins & Aggregates BFO: object aggregate protein aggregate amyloid plaque Lewy body amyloid aggregate CHEBI: macromolecule has part has part has part has part has part neurofibrillary tangle macromolecule aggregate PR: tau PR: amyloid-beta 42 PR: amyloid PR: amino acid chain PR: protein PR: ubiquitin PR: alpha-synuclein has part Aggregates & Diseases BFO: disposition ND: Lewy body disease ND: amyloidosis linked to ND: frontotemporal ND: Alzheimers dementia with disease Parkinsonism ND: tauopathy BFO: object aggregate linked to OGMS: disease protein aggregate amyloid aggregate Lewy body linked to macromolecule aggregate neurofibril ary tangle amyloid plaque linked to Aggregates, Processes, & Diseases BFO: object ag regate macromolecule ag regate protein ag regate produces GO: biol gical proces BFO: proces has part OGMS: disease course protein GO: protein ND: amyloidosi ND: tauopathy ag regation polymerization disease course disease course has particpant has particpant has part has part tau ag regation has particpant has particpant has part realizes realizes realizes realizes realizes realizes BFO: dispositon OGMS: disease ND: tauopathy ND: amyloidosi ND: fronto emporal ND: fronto emporal ND: Alzheimers ND: Lewy body neurofibrilary amyloid amyloid plaque GO: amyloid ND: Lewy body ND: Alzheimers dementia with dementia with disease disease tangle ag regate formation fibril formation disease course disease course Parkinsonism Parkinsonism disease course produces amyloid Lewy body plaque produces Aggregates & Processes BFO: object aggregate protein aggregate amyloid aggregate macromolecule aggregate neurofibril ary tangle produces GO: biological process BFO: process has part has participant has participant OGMS: disease course protein GO: protein ND: amyloidosis ND: tauopathy aggregation polymerization disease course disease course has part tau aggregation has participant has participant has part has part ND: frontotemporal amyloid plaque GO: amyloid ND: Lewy body ND: Alzheimers dementia with formation fibril formation disease course disease course Parkinsonism disease course produces amyloid Lewy body plaque produces Proteins, Aggregates, & Processes CHEBI: macromolecule BFO: object ag regate protein ag regate macromolecule ag regate has part has participant P R : a mi n o acid chain PR: protein has part has part has participant amyloid has part plaque has part produces produces produces Lewy body GO: biological proces BFO: proces has part OGMS: disease course protein GO: protein ND: amyloidosis ND: tauopathy ag regation polymerization disease course disease course has participant has participant has part has part tau ag regation has participant has participant has part ND: frontotemporal PR: tau neurofibril ary amyloid amyloid plaque GO: amyloid ND: Lewy body ND: Alzheimers dementia with tangle ag regate formation fibril formation disease course disease course Parkinsonism disease course PR: amyloid PR: ubiquit n PR: amyloid-beta 42 PR: alpha-synuclein has part has participant Proteins, Aggregates, Processes, & Diseases CHEBI:macromolecule P R : a m in o acidchain PR:protein haspart haspart hasparticpant haspart BFO:object ag regate macromolecule ag regate protein ag regate hasparticpant amyloid produces produces GO:biol gical proces BFO:proces haspart OGMS:disease c ou r s e protein GO:protein ND:amyloidosi ND:tauopathy ag regation polymerization diseasecourse diseasecourse hasparticpant hasparticpant haspart haspart tauag regation hasparticpant haspart realizes realizes realizes realizes realizes realizes BFO:dispositon OGMS:disease ND:tauopathy ND:amyloidosi ND:frontoemporal PR:tau ND:frontoemporal neurofibrlary amyloid amyloidplaque GO:amyloid ND:Lewybody ND:Alzheimers dementiawith ND:Alzheimers ND:Lewybody dementiawith tangle ag regate formation fibrlformation diseasecourse diseasecourse Parkinsonism disease disease Parkinsonism diseasecourse PR:amyloid PR:ubiquitn PR:amyloid-beta42 haspart produces L e w y bo d y PR:alpha-synuclein hasparticpant haspart plaque haspart hasparticpant Discussion: Linking Proteins to Diseases • ND’s asserted hierarchy groups neurological diseases based on the primary mechanism. In many cases, this is thought to be protein misfolding/aggregation (e.g. amyloidosis, tauopathy, alpha-synucleinopathy). Thus, ND already asserts a connection between certain diseases and proteins. • Is ‘OGMS: has material basis’ a shortcut relation between diseases and proteins? Is this what we want? Is it too strong or too weak? • Asserting protein participation in a disease course or pathological process is not an assertion of causality. Does this make participation too weak? • Do we need an explicitly causal relation? If so, what and how do we define it? Future Work • Finalize definitions for key terms and move relevant terms to PRO and GO • Add enhanced representations for additional protein aggregates • Represent additional disease courses and causal disease hypotheses involving protein aggregates • Identify additional methods for linking proteins and diseases • Refine the distinction between pathological and non-pathological protein aggregates Acknowledgments PRO Consortium Mark Jensen Patrick L. Ray Travis Allen Alan Ruttenberg Barry Smith Alexander D. Diehl Thanks to PRO Grant NIGMS 2R01GM080646-06 for project funding! ND publications and OWL file: https://code.google.com/p/neurological-disease-ontology/ Contact us: Alexander P. Cox - [email protected] Alexander D. Diehl - [email protected] (PI)