* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
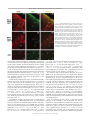
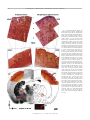
Download Basal Forebrain Cholinergic System Is Involved in Rapid Nerve
Nervous system network models wikipedia , lookup
Long-term depression wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Synaptogenesis wikipedia , lookup
Time perception wikipedia , lookup
Affective neuroscience wikipedia , lookup
Biology of depression wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Neuroanatomy wikipedia , lookup
Metastability in the brain wikipedia , lookup
Apical dendrite wikipedia , lookup
Neuroesthetics wikipedia , lookup
Microneurography wikipedia , lookup
Development of the nervous system wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Environmental enrichment wikipedia , lookup
Human brain wikipedia , lookup
Circumventricular organs wikipedia , lookup
Orbitofrontal cortex wikipedia , lookup
Aging brain wikipedia , lookup
Optogenetics wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Cortical cooling wikipedia , lookup
Neuroeconomics wikipedia , lookup
Spike-and-wave wikipedia , lookup
Synaptic gating wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Motor cortex wikipedia , lookup
Inferior temporal gyrus wikipedia , lookup
Neuroplasticity wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
J Neurophysiol 91: 424 – 437, 2004. First published September 24, 2003; 10.1152/jn.00489.2003. Basal Forebrain Cholinergic System Is Involved in Rapid Nerve Growth Factor (NGF)-Induced Plasticity in the Barrel Cortex of Adult Rats Neal Prakash,1 Susana Cohen-Cory,1 Silke Penschuck,1 and Ron D. Frostig1–3 Departments of 1Neurobiology and Behavior, 2Biomedical Engineering, and the 3Center for the Neurobiology of Learning and Memory, University of California, Irvine, California, 92697-4550 Submitted 21 May 2003; accepted in final form 15 September 2003 Prakash, Neal, Susana Cohen-Cory, Silke Penschuck, and Ron D. Frostig. Basal forebrain cholingergic system is involved in rapid nerve growth factor (NGF)-induced plasticity in the barrel cortex of adult rats. J Neurophysiol 91: 424 – 437, 2004. First published September 24, 2003; 10.1152/jn.00489.2003. We have previously reported that topical application of nerve growth factor (NGF) to the barrel cortex of an adult rat rapidly augmented a whisker functional representation (WFR) by increasing its area and height within minutes after NGF application. In addition, we found that TrkA, the highaffinity NGF receptor, was only found on fibers projecting into the barrel cortex. Here we use a combination of techniques including chronic intrinsic signal optical imaging, neuronal fiber tracking and immunohistological techniques, to test the hypothesis that NGF-induced rapid cortical plasticity is mediated by the cortical projections of the basal forebrain cholinergic system (BFCS). Our studies localize the source of the cells in the BFCS that project to a single WFR and also demonstrate that TrkA-immunoreactive fibers in the cortex are also cholinergic and likely arise from the BFCS. In addition, by selectively lesioning the BFCS cortical fibers with the immunotoxin 192 IgG-saporin, we show that NGF-induced WFR-cortical plasticity is eliminated. These results, taken together with our previously reported imaging results that demonstrated that agonists of the cholinergic system (particularly nicotine) showed transient NGF-like augmentations of a WFR, implicate the BFCS cortical projections as necessary for NGF’s rapid plasticity in the adult rat somatosensory cortex. Previously we reported that neurotrophins, such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), can rapidly induce large-scale plasticity in the adult somatosensory cortex (Prakash et al. 1996a,b). For example, within minutes after topical application to the cortex, NGF caused a transient, large-scale expansion in the area of a whisker functional representation (WFR) coupled with an increase in the height of the WFR. The current study attempts to describe potential underlying mechanisms that mediate this NGF-plasticity. One possibility may be that NGF is acting directly on cortical neurons to induce such plasticity. However, most evidence indicates that in the adult rat, NGF receptors are not expressed by cortical neurons but rather in neurons that project to the cortex (Merlio et al. 1992) and on projection fibers to the somatosensory cortex (Holtzman et al. 1995; Prakash et al. 1996b). This evidence thus suggests that NGFinduced adult cortical plasticity should involve neuronal projections into the cortex that are presynaptically responsive to NGF. Here we present evidence that indicates that the basal forebrain cholinergic system (BFCS) is the source of these cortical projection fibers and thus implicates the BFCS in the NGF-induced cortical plasticity. The basal forebrain (BF) in the rat, known as the magnocellular basal forebrain nuclei, includes cells located in the nucleus basalis of Meynert and the septal nucleus of the diagonal band of Broca (Fibiger 1982; Sofroniew et al. 1982; Wenk et al. 1980). Cholinergic cells within this system project to the entire rat cortex (Lehmann et al. 1980) in a loose topographically organized manner (Jimenez-Capdeville et al. 1997; Lamour et al. 1982) and constitute the only known cholinergic projection to the adult cortex (Mesulam et al. 1983). After release, acetylcholine (ACh) can influence target cells in the cortex through two broad classes of cholinergic receptors: muscarinic and nicotinic receptors. It has been demonstrated that NGF is synthesized in the cortex by the target cells of BF projections in the cortex and that it binds to receptors located on the BF nerve terminals and is retrogradely transported to cell bodies of BF neurons, where it regulates their survival and function as predicted by the neurotrophic hypothesis (Cuello et al. 1992; Hefti et al. 1989). While extensive evidence exists for the neurotrophic hypothesis in the developing brain, evidence also exists to support the notion that NGF continues to play a maintenance function for the BFCS after the brain matures. Exogenous application of NGF can rescue axotomized BF cholinergic neurons, reverse the decline in cortical function, and restore memory (Dekker et al. 1992; Gage et al. 1988; Jacobs et al. 1994; Koliatsos et al. 1990; Kromer 1987; Will and Hefti 1985; Williams et al. 1986). More direct experimental proof for the role of endogenous cortical NGF in the maintenance of the BFCS (Minger and Davies 1992a,b) was obtained when local injections of anti-NGF antibodies were delivered into the cortex of the adult rat (Gutierrez et al. 1997). These rats demonstrated a lack of ACh release in the cortex, a disruption of connectivity between cortex and BF, and a disruption of learning and memory. The importance of endogenous cortical NGF was also demonstrated by the use of mutant mice (Chen et al. 1997). Heterozygous mutant mice for the NGF gene showing a reduced level of both NGF-mRNA and protein within the cortex had significant learning and memory problems that were accompanied by a loss and shrinkage of BF cells. Infusion of NGF in these adult mutants abolished the memory deficits and corrected the def- Address for reprint requests and other correspondence: R. D. Frostig, Dept. of Neurobiology and Behavior, University of California, Irvine CA, 926974550 (E-mail: [email protected]). The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked ‘‘advertisement’’ in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. INTRODUCTION 424 0022-3077/04 $5.00 Copyright © 2004 The American Physiological Society www.jn.org MECHANISMS UNDERLYING NGF CORTICAL PLASTICITY icits in both area and projection density of BF cells to the cortex. It is clear that NGF plays a role in maintaining the BF cholinergic projections to the cortex. Two key findings have also suggested the possibility that NGF can also induce immediate release of ACh. Knipper and collaborators obtained in vitro evidence for NGF-induced rapid release of ACh in the cortex (Knipper et al. 1994a,b). The authors demonstrated such effects in synaptosomes prepared from the rat hippocampus that included BF projection fibers. They showed enhanced ACh release from these synaptosomes starting within 1 min after NGF application. Anti-NGF antibodies blocked the NGFinduced rapid release of ACh. The rapid induction of ACh release cannot be readily explained by NGF’s known role of regulating the survival and function of BF cholinergic system as this role requires a longer window of time (hours to days) for these effects. In addition, the rapid release of ACh supports the existence of NGF receptors on the terminals BF projection fibers. Rapid induction of ACh release by NGF was also demonstrated in synaptosomes prepared from the visual cortex of rats and further demonstrated that such induction of ACh release involves the activation of the high-affinity TrkA receptor and the p75 low-affinity neurotrophin receptor (p75) (Sala et al. 1998). Further, we obtained in vivo evidence for NGF’s ability to rapidly increase (within minutes after application) the height and areal extent of whisker evoked activity in the somatosensory cortex (Prakash et al. 1996b). Our previous histological results suggested that NGF’s site of action is not directly on the neurons within the somatosensory cortex but rather on projections originating outside it. Given that BFCS projection neurons are the only type of neurons with both projections to the cortex and NGF receptors (Holtzman et al. 1995), our findings (Prakash et al. 1996b) suggested an in vivo link for NGF’s rapid in vitro effects that may be mediated by the BFCS. One possible mechanism for NGF’s induced plasticity is that under behavioral conditions when cortical representations need to be rapidly augmented by increasing cortical excitability and by recruiting more neurons, cortical neurons release NGF in an activity-dependent manner in addition to the basal release of NGF needed for the survival and maintenance of the BF. The released NGF binds to its receptors (TrkA and p75) located on the BFCS projection fibers in the cortex. The binding of NGF then enhances the release of ACh from BFCS projections within the cortex. The release of ACh, in turn, further enhances cortical activity through ACh receptors and potentially glutamate release enhancement by ACh. These actions, in turn, lead to an increase in the excitability of neurons within a WFR and the recruitment of additional neurons to the WFR, leading an areal expansion and increased height of the WFR as observed with the imaging results (Prakash et al. 1996b). Elsewhere we reported, in support of this hypothesis, that activation of muscarinic or nicotinic receptors in the somatosensory cortex results in a WFR plasticity that is similar to the NGF-induced plasticity (Penschuck et al. 2002). Here we present data that tested three other critical aspects of our hypothesis: we demonstrate that projections from BFCS reach the level of a single WFR in barrel cortex; we demonstrate that TrkA-immunoreactive fibers present in the somatosensory cortex are also cholinergic fibers that likely arise from the BFCS; and we show that specifically lesioning the BFCS fibers in the barrel cortex J Neurophysiol • VOL 425 results in a loss of the ability of NGF to rapidly augment a WFR. Some of these data have been published previously in abstract form (Prakash et al. 2000a). METHODS Imaging We have followed a procedure described in Prakash et al. (1996b) and is described briefly here. Adult male Sprague-Dawley rats (300 – 550 g) were initially anesthetized with pentobarbital (50 mg/kg ip). Throughout the rest of the experiment, pentobarbital was injected as needed to maintain the rat in a consistent anesthetic state such that the respiration rate was 1.1 ⫾ 0.2 (range) breaths/s, rectal temperature was 37.0 ⫾ 0.5°C, ear color was pinkish-pale, and the corneal reflex in response to a saline drop was weak to mild. Lidocaine (0.1 ml) was injected under the scalp, and 5 min later a single incision was made with a scalpel roughly along the midline of the scalp. Fascia and muscle overlying the parietal cortex were cleared, and the bone was cleaned and dried. Next, the skull overlying the somatosensory cortex was removed. However, unlike the previous protocol (Prakash et al. 1996b) in which the dura and arachnoid mater were removed (durectomy), in this set of experiments, the dura remained in place but small slits or holes were made in it (durotomy) using a 30-gauge needle. This change in protocol was instituted to decrease the probability that rats would have significant brain herniation. A petroleum-jelly well was then built around the partially exposed cortex, filled with artificial cerebrospinal fluid [ACSF, containing (in g) 7.250 NaCl, 0.370 KCl, 0.170 KH2PO4, 0.277 CaCl2, 0.290 MgSO4, 2.160 NaHCO3, and 1.800 glucose (all per 1 l H20), pH 7.30, 37°C], and sealed with a coverslip. A 12-bit slow-scan CCD camera (Photometrics, Tucson, AZ) imaged a 6.8 ⫻ 5.1 mm (192 ⫻ 144 pixels; 35.5-m pixel width) region of the cortex that was illuminated with a stabilized 100-W tungstenhalogen light source (Zeiss Instruments) passed through an optical filter (630 nm band-pass 30 nm; Omega Optics, Brattleboro, VT). To reduce potential surface artifacts induced by pulsations of the brain and vessels, the camera was focused 300 m below the surface of the cortex. The light reflectance was measured for each pixel (9 consecutive frames, 500 ms each) before, during, and after stimulation of the contralateral whisker(s). Whisker stimulation was given with a computer-controlled mechanical stimulator (Bakin Systems II, Irvine, CA) at 5 Hz for 1 s. The interstimulus interval between trials was 16 s. Data files were created by the summation of 64 trials and thus had duration of ⬃20 min. The right C2 whisker was deflected 2° rostrocaudally (0.5 mm at 1.5 cm from the snout) with an average velocity of 30°/s. Eight imaging data files were collected per rat. The first four data files served as baseline reference. Additionally as an internal control to determine the affects of change the ACSF in the petroleumjelly well, the ACSF was replaced between data files 2 and 3. No significant difference in the measured parameters was ever noted after this ACSF change. After the fourth data file, the ACSF was replaced with recombinant human NGF dissolved in ACSF (15 g in 500 l). Quantitative analysis of a whisker functional representation Analysis methods have been described in detail elsewhere (ChenBee et al. 1996; Masino and Frostig 1996; Masino et al. 1993; Prakash et al. 1996b, 2000b). Briefly, data files were created from the summation of 64 imaging trials. Ratio values for a data file were computer-calculated for each pixel by dividing the reflectance values of the images 0.5–1.5 s poststimulation by the images 1.0 – 0.0 s before stimulation. Before analysis, a Gaussian filter (half-width 5) was applied to the raw ratio values to filter out high-frequency noise. The peak location of a WFR is defined as the pixel with the most negative ratio value, and this ratio value minus the median ratio value is the peak height of a WFR, which indicates the maximal magnitude of 91 • JANUARY 2004 • www.jn.org 426 N. PRAKASH, S. COHEN-CORY, S. PENSCHUCK, AND R. D. FROSTIG evoked activity. The area at half-height of a WFR is the area bounded by the threshold that contains pixels 50% of the peak height value. Relationship between imaging and underlying neuronal activity We focused our analysis on reflectance changes of 630-nm light occurring between 0.5 and 1.5 s poststimulus onset, an epoch of activity predominantly consisting of localized oxygen consumption in the capillary bed serving activated neurons (Ances et al. 2001; Frostig et al. 1990; Kim et al. 2000; Malonek and Grinvald 1996; Malonek et al. 1997; Thompson et al. 2003) as the area of imaged activity generated from this epoch has been previously found to be well correlated with the underlying area of evoked neuronal activity (BrettGreen et al. 2001; Frostig et al. 1990; Masino 2003; Polley et al. 1999). We also have repeatedly found a strong correspondence between the spatial distribution of intrinsic signals and the underlying single-unit responses such that the location of the strongest single-unit response for each rat coincided with the location of that rat’s strongest intrinsic signal response; evoked single-unit responses were present at all locations containing evoked intrinsic signal responses; and both responses decayed similarly with increasing distance from the location of peak activity (Brett-Green et al. 2001; Masino 2003; Polley et al. 1999). Furthermore, postimaging single-unit recordings demonstrated that plasticity of a WFR (both in its areal extent and height) was identical to the one obtained with intrinsic signal optical imaging (Polley et al. 1999). Thus for both the normal functional organization of cortex and its plasticity, intrinsic signal optical imaging that is based on localized oxygen consumption has been shown to be a reliable indicator of underlying physiological neuronal activity patterns and their plasticity. Retrograde labeling of basal forebrain cells projecting to barrel cortex Fifteen male Sprague-Dawley adult rats (300 – 600 g) were imaged, and the exact location of the peak of activity for the C2 WFR was determined using stereotactic methods. The location of Bregma was defined as the origin, and C2 WFR location was measured in relation to the origin using its peak activity as obtained by imaging through the thinned skull (⬃150 m thickness). The average location of C2 was useful in other experiments, such as the 192-IgG-saporin injections described in the following text, and in localizing the C2 barrel in coronal sections. A small burr hole was drilled through the cortex over the peak activity of the WFR. Approximately 1 l Fast-DiI oil (1,1⬘-dilinoleyl-3,3,3⬘,3⬘-tetramethylindocarbocyanine perchlorate, Molecular Probes, Eugene, OR) dissolved in dimethylsulfoxide was then pressure injected (PicoSpritzer II, General Valve, Brookshire, TX) through a glass pipette that was lowered 500 m below and perpendicular to the pial surface. Fast-DiI is a fluorescent molecule that travels along lipid membranes, thus staining axons (anterogradely and retrogradely) and cell bodies (predominantly retrogradely). After waiting 3 min, the pipette was slowly retracted, and the burr hole was sealed with sterile bone wax. The scalp incision was closed with wound clips, and prophylactic antibiotics were administered (Panalog (nystatin, neomycin sulfate, thiostrepton, triamcinolone acetonide) on the eyes, Terramycin over the wound, and Ampicillin subcutaneously in the back). Rats were survived for 6 –10 days and then given an overdose of sodium pentobarbital (Nembutal, 100 mg). When completely areflexive, rats were perfused transcardially with 0.1 M PBS at 4°C (⬃150 ml) followed by fixation with a solution containing 4% paraformaldehyde and 0.25% gluteraldehyde in 0.1 M Sorensen’s buffer (⬃150 ml). The brain was removed and postfixed in the same solution overnight, followed by immersion for 2–3 days in 30% sucrose dissolved in 0.1 M PB. On sinking, the brain was cut coronally on a cryostat at –20°C; each section was thaw-mounted serially directly onto gelatin-coated slides. As DiI is prone to dissolution after J Neurophysiol • VOL sectioning, sections were viewed either dry or with 0.1 M PBS and a coverslip. The locations of DiI-positive cell bodies throughout the basal forebrain were noted and photographed using a Rhodamine filter on a fluorescent microscope (Nikon). Techniques to detect cholinergic neurons and fibers Cholinergic neurons in the nervous system produce the neurotransmitter acetylcholine by the enzyme choline acetyl transferase (ChAT). ACh is then transported into synaptic vesicles by the vesicular acetylcholine transporter (VAChT). After acetylcholine is released at the synapse, it is rapidly degraded by acetylcholine esterase (AChE). Based on these three enzymes, different histochemical methods have been developed to visualize cholinergic neurons. AChE visualization techniques are the oldest and best-characterized staining techniques for cholinergic neurons and fibers. These techniques are the mostsensitive visualization techniques but are not specific enough because norepinephrinergic and dopaminergic neurons may also stain positive for AChE (Albanese and Butcher 1979; Henderson 1989; Tago et al. 1986). Immunohistochemical techniques for staining neurons and fibers for either ChAT of VAChT have proven to be more specific for cholinergic neurons but are generally less sensitive at elucidating cholinergic fibers than AChE staining (Armstrong et al. 1983; Eckenstein and Sofroniew 1983; Holler et al. 1996; Mizukawa et al. 1986; Satoh et al. 1983; Weihe et al. 1996). Therefore depending on whether we required sensitivity or specificity, all three staining methods were chosen for different experiments described in the following text. Co-localizing TrkA and ChAT We required maximal specificity to demonstrate that cholinergic fibers were also expressing TrkA. Therefore ChAT was chosen for these experiments. Initial studies were conducted to optimize the single staining protocols for TrkA and ChAT, and in few rats, double staining with VAChT and TrkA was also performed. A rat was given an overdose of pentobarbital and then perfused transcardially with 4°C PBS (0.1 M PBS, ⬃150 ml) and fixed with 4% paraformaldehyde (⬃150 ml). The brain was removed and placed in the same fixative overnight, then transferred to 30% sucrose in 0.1M PB until it sank (⬃3 days). The brains were frozen (⫺20°C) and sectioned to 35-m slices. For double staining, 20- to 35-m slices were incubated simultaneously with goat anti-ChAT antibody (Chemicon International, Temecula, CA) and rabbit anti-TrkA antibody (Holtzman et al. 1995) in 5% donkey serum overnight at 4°C. After rinse in 0.1 M Tris-buffered saline (TBS), sections were incubated for 1 h at room temperature in darkness in Cy2-conjugated donkey anti-rabbit antibody and Cy3-conjugated donkey anti-goat antibody (both from Jackson ImmunoResearch Labs). Four controls were used that had identical treatment as the other slices except for the omission of one of the following antibodies: anti-ChAT, anti-TrkA, anti-rabbit, or anti-goat. After rinse in 0.1 M TB, sections were mounted on gelatin-coated slides and dried for ⬃15 min. Sections were treated with Prolong mounting media (Molecular Probes) and coverslipped and stored in the dark at 4°C. One to 2 days later sections were visualized and imaged under a fluorescent microscope (Nikon or Olympus) with a FITC (for Cy2) or rhodamine (for Cy3) filter. Cholinergic depletion of a cortical whisker functional representation IgG-saporin (192) is a cytotoxin that was developed by R.G. Wiley and colleagues and has been demonstrated to be extremely effective and specific at eliminating cholinergic basal forebrain cells (Book et al. 1992, 1994; Sachdev et al. 1998; Wenk 1997; Wiley 1992; Wiley et al. 1991) (Baskerville et al. 1997; Zhu and Waite 1998). IgGsaporin (192) works by binding to p75 receptors on cholinergic fibers, where it is internalized and transported back to the nucleus. Saporin 91 • JANUARY 2004 • www.jn.org MECHANISMS UNDERLYING NGF CORTICAL PLASTICITY then binds to ribosomes and irreversibly blocks protein synthesis, which leads to cell death. One effective method of depleting the barrel cortex of cholinergic inputs is by ventricular injection of 192 IgGsaporin (Baskerville et al. 1997; Zhu and Waite 1998; reviewed by Schliebs et al. 1996; Wiley 1996). However, even more precise, localized depletions of cholinergic inputs are possible by injection of a small amount of 192 IgG-saporin directly into the cortex (Bucci et al. 1998; Sachdev et al. 1998). Data obtained from such localized injections is not confounded by interpretations related to possible secondary effects from the complete cholinergic depletion of a hemisphere(s) and parts of the cerebellum, as obtained with intracerebroventricular injections (Wiley et al. 1991). To test the hypothesis that cholinergic fibers are involved in NGF’s induced plasticity of a WFR, we locally depleted cholinergic fibers within the C2-WFR, using a similar protocol as Sachdev and collaborators who were also using 192 IgG-saporin to test cortical plasticity in the adult barrel cortex (Sachdev et al. 1998). These authors reported that local injection of 192 IgG-saporin into the barrel cortex results in a loss of the normal cortical plasticity that occurs between two-whiskers when all the other whiskers are trimmed from a rat’s snout (Sachdev et al. 1998). Combining chronic imaging with cholinergic depletion Thirty rats were used in this study, 20 experimental and 10 controls. Of the 20 experimental rats, 8 were successful for all 3 phases described in the following text. Additionally 3 of the remaining 12 experimental rats were successful in the first two phases but had only negligible AChE fiber depletion (⬎75% remaining) in the C2-barrel column, as evident in phase 3. For the control rats, 6 of 10 were successful for all 3 phases. The right C2 WFR was imaged through a thinned skull in pentobarbital-anesthetized adult male Sprague-Dawley rats (300 –550 g). To avoid direct damage to the C2 WFR, a burr hole in the skull was drilled between 1.5 and 2.0 mm just medial of the peak of activity of the C2 WFR. Eight nanograms 192 IgG-saporin (Chemicon) dissolved in 2 l 0.1 M PBS with 0.05% sodium azide (or only 2 l of PBS and 0.05% sodium azide for controls) was pressure-injected (Picospritzer II, General Valve) through a glass pipette 1,500 m below the pial surface. Similar to (Sachdev et al. 1998); this depth allowed for good penetration and diffusion of the toxin throughout the cortical tissue. PHASE 1—LOCALIZATION OF WFRS AND INJECTION. J Neurophysiol • VOL 427 Also similar to Sachdev et al. (1998), the distance of 1.5–2.0 mm from the C2 WFR was found to be close to the maximal distance for inducing an effective lesion in C2 in this dosage range. This distance also prevented direct damage to the C2 WFR from the pipette. Additional care was taken to avoid traumatizing major cortical vasculature with the pipette. Once injected, the pipette was slowly retracted, and the burr hole was sealed with sterile bone wax. The scalp incision was closed with wound clips, and prophylactic antibiotics were administered (Panalog on the eyes, Terramycin over the wound, and Ampicillin subcutaneously). Rats recovered from surgery overnight in a cage resting on a heated blanket, and then were returned to individual cages in the vivarium for 21 days with free access to food and water on a 12-h light, 12-h dark cycle. PHASE 2—NGF-EFFECTS ON DEPLETED AND CONTROL CORTEX. Similar to Sachdev et al. (1998), 21 days was chosen as the interval between injection and second imaging of the C2 WFR; this interval allowed for a full recovery from the surgery. Twenty-one days after the first imaging session/injection, rats were briefly observed for gross behavioral changes and then weighed. All rats gained between 5 and 100 g over the 21 days, and no gross behavioral changes were observed. Rats were then anesthetized with intraperitoneal pentobarbital and local lidocaine and placed in the stereotactic apparatus. The remaining wound clips were removed, and the incision site re-opened. The “cortical window” in the skull that from the previous imaging session was frequently revascularized and thus required cleaning and cauterizing. Once cleaned, the cortical window was resected using a dental drill and forceps. To allow access of NGF into the cortical parenchyma, in all rats, three to four small slits (⬃100 ⫻ 1,500 m each; Fig. 8) were made in the dura and arachnoid mater using a 30-gauge needle. The slits were made in locations that surrounded and touched the borders of the previously identified location of the C2 WFR and that avoided tearing the dural vessels. A petroleum-jelly well was then built on the bone surrounding the cortical window and filled with ACSF and topped with a coverslip. The imaging protocol as described in the preceding text and previously (Prakash et al. 1996b) was then used to assess the C2 WFR before and after application of NGF. PHASE 3—QUANTIFYING THE CHOLINERGIC DEPLETION. At the end of the imaging session, rats were perfused transcardially with 4°C 0.1 M PBS (⬃150 ml) followed by fixation with a solution of 4% paraformaldehyde, 0.5% gluteraldehyde, and 0.2% picric acid in 0.1 91 • JANUARY 2004 • www.jn.org 428 N. PRAKASH, S. COHEN-CORY, S. PENSCHUCK, AND R. D. FROSTIG M Sorensen’s buffer (⬃150 ml). The brain was removed and placed in 30% sucrose in 0.1 M PB until it sank. (Approximately half the brains were prepared for tangential cutting: the cortex was dissected from the rest of the brain and flattened to 2.0 mm between 2 slides and then the slides and brain were placed in 30% sucrose). The brains were then frozen to –20°C, and serial sections were cut coronally or tangentially at 40-m thickness on a cryostat (Zeiss). Alternating sections were stained for cytochrome oxidase (CO) (Wong-Riley 1979) to visual barrels and layer IV. The other alternating sections were stained for AChE, using Tago’s modifications (Tago et al. 1986). Additionally, slices were obtained from several rats and stained for recombinant human NGF-immunoreactivity to ascertain the penetration of NGF through dural tears. This protocol was similar to as previously described (Prakash et al. 1996b). Sections were mounted onto gelatin-coated slides and allowed to dry, then covered with mounting media followed by coverslips. Sections were analyzed and imaged under a microscope (Olympus), and the location of the left and right C2 barrels and their relations to the vascular patterns were ascertained from the CO sections. The vascular patterns from the adjacent AChE section were then compared with CO section vascular patterns and used to determine the location of the C2-barrels in that section. Photomicrographs (⫻20 or ⫻40) were then taken in each C2-barrel and in layer I/II directly above the barrel. Photomicrographs were digitized, and in PhotoShop 5.0 (Adobe, San-Jose, CA), a 100-m line was then drawn through the barrel in layer IV and above the barrel in layer I/II. The line was drawn perpendicular to the pial surface for coronal sections or horizontal for tangential sections. “Fiber crossings” were calculated by adding up the number of intersections the two 100-m lines made with AChE-positive fibers. AChE fiber depletion was defined as {1 ⫺ [(total fiber crossings on injected side)/(total fiber crossings on control side)] ⫻ 100%}. Although there were no obvious differences across the different lamina, we quantified the fiber depletion in layers IV and layer I/II as these layers are presumably the primary loci relevant to NGF’s effects. However, there were differences in the fiber depletion in the C2 barrel across different rats. This was most likely from the 1.5–2.0 mm variability in injection site distance from the C2 barrel, which was introduced to avoid damaging major blood vessels (see Phase 1 in the preceding text). However, this variability across rats proved to be advantageous because it allowed for a correlation of NGF’s induced plasticity with the fiber depletion ratio (Fig. 4). RESULTS Regional topography of basal forebrain projection to a WFR To determine whether the general pattern of BFCS projection cells to the somatosensory cortex holds even for a single WFR, we injected Fast-DiI, a retrograde tracer, into the cortical C2-whisker functional representation that resulted in transport of tracer to several areas of the brain, such as the thalamus, zona incerta, and basal forebrain, in similar patterns as we reported in mice (Prakash et al. 2000b). Previous studies have suggested that the somatosensory cortex of the rat receives basal forebrain projections predominantly from the nucleus basalis of Meynert [nBM; which consists of the ventral part of the globus pallidus (vGP), the substantia innominata (S.I.), some cells in the internal capsule, and a few cells scattered in other nuclei (Bigl et al. 1982; McKinney et al. 1983)]. Our primary interest in this study was to determine the precise regions of the basal forebrain that were labeled. First we wanted to verify the identities of the basal forebrain nuclei that project to the adult rat barrel cortex. Second, we wanted to determine whether an individual WFR had a more specific basal forebrain projection locus than reported for the rest of the J Neurophysiol • VOL somatosensory cortex (Bigl et al. 1982; Kiss and Patel 1992; McKinney et al. 1983). Consistent with these previous reports, which examined the innervation of the entire somatosensory cortex, we found that the C2-WFR was innervated by a scattered group of large cells throughout the nBM (Fig. 2). Other cells were found scattered throughout the basal forebrain but never in as high a concentration as the nBM. Overall, our results suggest that the pattern of projections to a single WFR is not more localized than the general pattern of BF cells that project to the sensory cortex. These results were helpful for the interpretation of the intracortical 192 IgG-Saporin injections described in the following text. Cytochemistry of basal forebrain projection cells and TrkA cortical fibers The basal forebrain contains at least two major classes of neurons that project fibers to the cortex: parvalbumin-immunoreactive (-IR; GABAergic) and ChAT-IR (cholinergic). The cholinergic fibers are the best candidates for also expressing TrkA because previous work demonstrated that most of the abundant ChAT-IR cell bodies in the basal forebrain are also p75-IR/TrkA-IR (Sobreviela et al. 1994), whereas GABAergic cells in the basal forebrain are p75-IR negative (Kiss et al. 1993). Although there is no question that cholinergic basal forebrain cells also express TrkA, there was no direct evidence that cholinergic projection fibers in the cortex co-express ChAT and TrkA. To assess whether TrkA-IR fibers observed in the cortex are also cholinergic, co-localization studies with TrkA-IR and ChAT-IR were performed. We found that TrkA fibers were less abundant than ChAT fibers (⬃1:4 ratio), but that all TrkA-IR fibers in the barrel cortex were also ChAT-IR (Fig. 1). Furthermore, the TrkA fibers were sparsely distributed, but present in all cortical layers (I–VI). To further verify this finding, double-labeling studies with TrkA-IR and the vesicular acetylcholine transporter (VAChT)-IR were performed (not shown). These experiments demonstrated similar types of staining patterns as the ones presented in Fig. 1. In summary, we found TrkA-IR only on fibers in the adult rat barrel cortex, and all these fibers also were cholinergic (either ChAT-IR or VAChT-IR). Necessity of the bfcs for the expression of ngf’s rapid plasticity Confirming our observation that TrkA expression is found exclusively in projection fibers into barrel cortex and that all TrkA-IR fibers are cholinergic, we next analyzed the results of depletion of the BFCS fibers in the barrel cortex (Fig. 2). This depletion was accomplished by injecting 192 IgG-saporin into the cortex near the C2-WFR and then re-imaging of the same C2-WFR 3 wk later. Because of our histological studies using Fast-DiI, we knew that the pattern of BF projection cells to a single WFR is similar to the general patterns of BF projection cells to the somatosensory cortex. Thus imaging-based localized injections of 192 IgG-saporin to the somatosensory cortex near a single WFR would result in confined, yet representative and thus interpretable, pattern of lesions in the BF. In these rats the depletion was between 35 and 95%. A control group had saline injections into the cortex near the C2-WFR, which had 91 • JANUARY 2004 • www.jn.org MECHANISMS UNDERLYING NGF CORTICAL PLASTICITY 429 FIG. 1. TrkA-immunoreactive (IR) and choline acetyl transferase-IR (ChAT-IR) co-localize in nucleus basalis of Meynert cells and in cortical fibers. Thin sections of a rat brain were double stained with specific antibodies to ChAT and TrkA. Top: layer I of the barrel cortex. ChAT-IR fibers are present near the pial surface of the cortex (top right). Of these fibers, a few are also immunopositive for TrkA (top middle), as shown by the overlaid image (yellow; see ). Middle: TrkA-IR fibers found in layer V of the barrel cortex. While ChAT and TrkA immunoreactivities are localized to individual fibers (), their localized distribution within the fiber is not necessarily overlapping/restricted to the same area. Bottom: immunostaining of a region of the substantia innominata in adult rat nucleus basalis of Meynert (nBM; coronal section) shows large ChAT-IR cells that are also immunopositive to TrkA. These images are typical of all rats examined, and similar results were obtained using an antibody to vesicular acetylcholine transporter. no detectable AChE fiber depletion. Additionally, a third group of rats had injection of 192 IgG-saporin that was subsequently found to have minimal AChE fiber depletion at the C2-WFR (between 0 and 25%). Both the control group (n ⫽ 6) and the group with an AChE fiber depletion between 0 and 25% (n ⫽ 3; Fig. 4) showed increases in both the area at half-height and the peak height after NGF application that were not statistically significantly different from each other (P ⬎ 0.05, t-test, n ⫽ 9). We thus pooled the control group and the group with AChE fiber depletion between 0 and 25% group together as the “non-depleted group” (n ⫽ 9). To determine if depleting the cortex of the BFCS affected a WFR 21 days after 192 IgG-saporin injection, we compared the area and strength of the C2-WFRs before application of NGF in the depleted and non-depleted groups. There was no difference between the C2-WFR of depleted and non-depleted groups for either the area at half-height [1.38 ⫾ 0.16 vs. 1.25 ⫾ 0.13 (SE) mm2, P ⬎ 0.05, t-test, n ⫽ 17] or the peak height (2.64 ⫻ 10⫺4 ⫾ 0.25 ⫻ 10⫺4 vs. 2.39 ⫻ 10⫺4 ⫾ 0.46 ⫻ 10⫺4, P ⬎ 0.05, t-test, n ⫽ 17). Therefore injection of 192 IgGsaporin locally into the cortex did not have a significant effect on a WFR when compared across rats (Fig. 6, also compare gray bars in Fig. 7). To determine whether topical application of NGF-induced WFR plasticity in a BFCS-depleted cortex, we examined the area and strength of the C2-WFR before and after NGF application. In the depleted group, the C2-WFR did not change significantly after topical application of NGF, for either area at half-height (1.38 ⫾ 0.16 vs. 1.33 ⫾ 0.13 mm2, P ⬎ 0.05, paired t-test, n ⫽ 8) or the peak height (2.64 ⫻ 10⫺4 ⫾ 0.25 ⫻ 10⫺4 vs. 2.61 ⫻ 10⫺4 ⫾ 0.30 ⫻ 10⫺4, P ⬎ 0.05, paired t-test, J Neurophysiol • VOL n ⫽ 8). In contrast, in the non-depleted group both the area at half-height (1.25 ⫾ 0.13 vs. 1.59 ⫾ 0.14 mm2, P ⬍ 0.05, paired t-test, n ⫽ 9) and the peak height (2.39 ⫻ 10⫺4 ⫾ 0.46 ⫻ 10⫺4 vs. 3.31 ⫻ 10⫺4 ⫾ 0.55 ⫻ 10⫺4, P ⫽ 0.01, paired t-test, n ⫽ 9) of the C2-WFR increased significantly after topical application of NGF (see Fig. 3 “augmentation” for the depiction of this specific plasticity; see also Fig. 4). To determine that the NGF-induced plasticity of the C2WFR in the non-depleted group was significantly different from the lack of change in the C2-WFR induced by NGF in the depleted group, we also compared changes in the area at half-height and peak height between depleted and non-depleted rats (Figs. 5 and 6). The change in the area at half height of the C2-WFR after NGF application was significantly larger in non-depleted versus depleted rats (36.7 ⫾ 11.2% vs. ⫺0.4 ⫾ 7.5%, P ⬍ 0.05, t-test, n ⫽ 17). Likewise, the change in the peak height of the C2-WFR after NGF application was significantly larger in non-depleted versus depleted rats (37.6 ⫾ 10.6% vs. 0.5 ⫾ 8.4%, P ⬍ 0.05, t-test, n ⫽ 17). In summary, if the cortex was depleted of ⬎35% of BFCS fibers within the C2-WFR, then topical application of NGF to the cortex did not induce the rapid plasticity of the C2-WFR observed in normal, non-depleted cortex. Last, we examined the time course of the NGF-induced plasticity of the C2-WFR in the non-depleted rats. For the depleted rats, topical application of NGF did not result in any significant changes in either the area at half-height or the peak height at any time bin measured (ⱕ100 min postapplication, 1-way repeated-measure ANOVA, n ⫽ 8; Fig. 7). For the non-depleted rats, both the area at half-height and the peak height of the WFR were significantly different from preappli- 91 • JANUARY 2004 • www.jn.org 430 N. PRAKASH, S. COHEN-CORY, S. PENSCHUCK, AND R. D. FROSTIG FIG. 2. Retrograde labeling studies and cholinergic fiber depletion quantification. Bottom: DiI injection into the supragranular layers of the C2-whisker functional representation yields retrogradely labeled cells predominantly in the ipsilateral nBM. Coronal section located –1.4 mm posterior from Bregma. Right: schematized; left: stained for acetylcholine esterase [AChE; brain atlas picture: adapted from (Paxinos and Watson (1986)]. The location of the DiI injection site within the C2-whisker functional representation (WFR) supragranular layers is depicted as a red spot under the red arrowhead (shown in a color coronal section stained for CO located ⫺3.0 mm posterior from Bregma). Red dots grossly schematize that the ventral portion of the globus pallidus (GP) had the highest density of retrogradely labeled cells; however, similar cells were seen just ventral in the substantia innominata (S.I.) as well as a few scattered cells nearby in the internal capsule (IC). This cell cluster between GP, IC, and S.I. is also known as the nBM. Inset: a photomicrograph of a DiI-labeled cell body (diameter: 20 m) in the nBM after DiI injection. Middle: composite images of coronal sections stained for AChE for control cortex (left) and 192 IgG-saporin injected cortex (right) from one rat at ⫻10 magnification showing the cortical layers I–VI. Note that typically AChE staining revealed fibers 5 times as abundant as ChAT-IR fibers and 15 times as abundant as TrkA-IR fibers (compare with Figs. 1 and 4). These slices were adjacent slices the CO-stained slices (see bottom), which enabled the depiction of the location of the C2 barrel (green outline). Top: [times}20 magnification of the same slices from middle. Each yellow dot overlies a fiber crossing (dots enlarged for visibility in this figure) through 100-m lines drawn in layer I and layer IV above and within the C2-barrel. Right: the AChE-fiber-depleted cortex after local injection of 192 IgG-saporin. In this case, the AChE-fiber depletion was 80%. J Neurophysiol • VOL 91 • JANUARY 2004 • www.jn.org MECHANISMS UNDERLYING NGF CORTICAL PLASTICITY 431 FIG. 3. Depiction of potential plasticity of a WFR. A WFR can change in area and/or height. The central 3-dimensional-rendered image is a typical pattern of activity for a normal WFR with green depicting baseline activity and the red peak depicting the maximal activity. A WFR can change after manipulations that induce cortical plasticity. For example, the top right image depicts an increase in height and area of a WFR, which we term “augmentation,” such plasticity was observed after NGF application (Prakash et al. 1996a,b) and nicotine application (Penschuck et al. 2002). Other types of plasticity have also been observed in WFRs, such as an “attenuation” after BDNF application (Prakash et al. 1996a,b) and an “expansion” after carbachol application (Penschuck et al. 2002). cation baseline at the time bin of 26 –50 min in the presence of NGF (1-way repeated-measure ANOVA, post hoc Dunnett’s test, P ⬍ 0.05, n ⫽ 9; Fig. 7). After this time bin, the values remained elevated but were not statistically different from baseline. In general, the timecourse of NGF’s plasticity of a WFR appears to be somewhat slower in rats with durotomies reported here versus rats with durectomies previously reported (Prakash et al. 1996b). The slower time course of the NGFinduced plasticity of a WFR may be related to a reduced amount of NGF reaching layer 1 through the durotomies compared with durectomies and/or a longer amount of time required to attain a sufficient concentration of NGF within layer 1. Detection of exogenous NGF penetration to the cortical tissue To determine how well NGF penetrated the cortex through the dural slits and holes (durotomies), NGF-IR was assessed in five rats (Fig. 8). We found that NGF-IR was detected in layer I (40 – 80 m), and thus NGF-IR was detected at a depth that was about one-third the depth of preparations previously reported with durectomies (Prakash et al. 1996b). Additionally, NGF-IR was generally localized in the cortex to the site diJ Neurophysiol • VOL rectly under the slits in the dura. It is likely that NGF spread beyond the dural slits, but low-level NGF-IR throughout the pial layer all over the brain obscured our ability to quantify immunoreactivity at depths of less than ⬃25 m below the pial surface. DISCUSSION Summary of results Several experiments were reported here that tested the hypothesis that the BFCS is involved in mediating NGF’s rapid cortical plasticity. First, we found that the projections of the BFCS can target a single WFR in the cortex and are mostly located in the nBM. Second, we found that TrkA-IR fibers are colocalized with ChAT-IR fibers in the adult rat barrel cortex that likely arise from the BFCS and therefore that by binding to TrkA, NGF has the potential to modulate acetylcholine release from the BFCS. Finally, we found that depletion of ⬎35% of the AChE fibers that innervate the C2-WFR prevented the rapid NGF-induced plasticity of the C2-WFR after topical application of NGF to the cortex. Overall, these experiments implicate the BFCS as necessary for NGF-induced cortical plasticity. 91 • JANUARY 2004 • www.jn.org 432 N. PRAKASH, S. COHEN-CORY, S. PENSCHUCK, AND R. D. FROSTIG recent study on the role of NGF in plasticity of the developing visual cortex led to the same conclusions (Rossi et al. 2002). In this study, cholinergic afferents to the visual cortex were destroyed by application of the excitotoxic drug quisqualic acid to the BF. Afterward, using Western blot, TrkA and p75 expression was dramatically but not completely reduced in the cortex, suggesting that in the developing rat these receptors are located on predominantly cholinergic terminals. Taken together, these results generalize the anatomical connections between NGF and the BFCS to both the developing and the adult cortex. NGF/basal forebrain cholinergic system functional interactions FIG. 4. Depleting the cortex of ⬎35% of AChE fibers prevents NGFinduced augmentation. Top: 3 wk after cortical 192 IgG-saporin injection and immediately after the imaging session, AChE fiber depletion was calculated by counting fiber crossings (see Fig. 2). This graph depicts the average number of fiber crossings in layer 1 and 4 for the uninjected control hemisphere and the 192 IgG-saporin injected hemisphere. {, the 6 rats with ⬎35% AChE fiber depletion; }, the 3 rats that had 0 –25% depletion. Bottom: this graph depicts that in cortex with AChE fiber depletion of ⬍25%, NGF application augmented a WFR (}), whereas in cortex with AChE fiber depletion ⬎35%, NGF application did not augment a WFR ({). One way to test whether the BFCS is implicated in NGF’s plasticity of a WFR is to directly lesion the BFCS with a specific toxin, such as 192 IgG-saporin, to detect whether NGF can still augment a WFR in the lesioned rat. An alternative way is to pharmacologically block the cholinergic receptors located on the cortical BFCS projections by topical application of a general cholinergic antagonist and detect whether NGF can still induce rapid plasticity of a WFR in such a pharmacologically blocked cortex. Unfortunately, there is no single general antagonist for both muscarinic and nicotinic cholinergic receptors. Therefore to perform properly such an experiment, at least two general antagonists are needed prior to the application of NGF. This is a complicated experiment because the time courses of the antagonists actions after topical application to the cortex is different as we previously demonstrated for the topical application of muscarinic and nicotinic receptor agonists (Penschuck et al. 2002). Thus a general cholinergic blockade of the cortex is challenging, and therefore the interpretation of the effect of NGF application is questionable under such conditions (Penschuck et al. 1999). Cytochemistry of basal forebrain projection cells and TrkA cortical fibers The basal forebrain contains at least two major projection neurons that have fibers in the cortex: IR (GABAergic) and ChAT-IR (cholinergic). The cholinergic fibers are the best candidates for also being the TrkA-IR fibers we observed in the barrel cortex, because previous work has shown that most of the abundant ChAT-IR cell bodies in the basal forebrain are also p75-IR/TrkA-IR (Sobreviela et al. 1994). While there is no question that BFCS cells also express TrkA, there is still no direct evidence that their projection fibers in the cortex coexpress ChAT and TrkA. This lack of direct evidence may be related to a low sensitivity of TrkA antibodies (Sobreviela et al. 1994) and even some ChAT antibodies at detecting antigen in the relatively thin cholinergic fibers. Nevertheless as we were able to detect TrkA fibers in the cortex, co-localization studies with TrkA and ChAT (or VAChT) were then performed. We found that while TrkA-IR fibers were less abundant than ChAT fibers, they were also ChAT-IR (or VAChT; Fig. 1). Because TrkA-IR fibers were found to be ChAT-IR (or VAChT-IR), this provided direct anatomical evidence that NGF can act on cholinergic fibers in the barrel cortex. It is interesting that a J Neurophysiol • VOL FIG. 5. NGF induces significant plasticity of a WFR in nondepleted cortex (dark triangles) but not in AChE depleted cortex (white dots). Small symbols represent changes from individual rats, whereas large symbols depict the averages and SE for the 2 groups. These 2 groups were statistically different from each other both for area at half-height and for peak height (see text for details). 91 • JANUARY 2004 • www.jn.org MECHANISMS UNDERLYING NGF CORTICAL PLASTICITY 433 FIG. 6. NGF induces significant plasticity of a WFR in normal cortex (■, F, }, Œ, Š, ) but not in AChE depleted cortex (䊐, E, {, ‚, ƒ). These graphs depict the actual values, not percent normalized (as in Fig. 5), for the peak height and area at half-height values in individual rats before and after NGF in AChE-depleted and non-depleted cortex. Dark lines depict the average trend for each graph. Using the 192 IgG-saporin lesion method described in the preceding text, we found that in rats with depletion of ⬎35% of the AChE fibers that innervate the C2-WFR, topical NGF application did not induce WFR plasticity, thus supporting the FIG. 7. Time course of NGF’s plasticity in AChE-depleted vs. non-depleted cortex. 1, the average area at half-height (top) or peak height (bottom) of a WFR before NGF application. 䊐 and ■, the averages at successive time bins in the continual presence of NGF. In cortex with depleted AChE fibers (left, 䊐) NGF did not increase either the area at half height or the peak height even after 100 min of application. In contrast, in non-depleted cortex (right, ■), NGF significantly increased the area at half-height and peak height ⬃30 min after its topical application to the cortex. Error bars denote SE; *, P ⬍ 0.05 vs. preapplication time bin. J Neurophysiol • VOL hypothesis that the BFCS is necessary for the mediation of NGF-induced rapid plasticity of a WFR. Additional support for functional interactions between NGF and the BFCS has been suggested from research on the developing visual cortex of the rat. Pesavento and colleagues have demonstrated in the developing rat visual cortex that NGF modulation of LTP is mediated by the cholinergic system (Pesavento et al. 2000). In addition, NGF-induced increase of ACh and glutamate release from cortical synaptosomes was strongly impaired after elimination of the cholinergic afferents to the visual cortex (Rossi et al. 2002). Taken together, these results generalize the functional interactions between NGF and BFCS as part of an underlying system important for cortical plasticity in both the developing and the adult cortex. This conclusion, however, seem at odds with a recent study that used transplants of NGF-secreting fibroblasts to restore a reduction stimulusevoked activity in barrel cortex of the adult rat (Rahimi and Juliano 2001). These authors demonstrate that NGF secreted from the grafted fibroblasts could restore a reduction of evoked-activity in a barrel cortex depleted from cholinergic projections from the BFCS. The restoration of stimulus-evoked activity happened in spite of the fact that the cortex was depleted of ⬎80% of AChE. These observations led the authors to conclude that NGF can directly affect cortical activity without the presence of the cholinergic system. The explanation for the apparent difference in conclusions may relate to many technical differences on how the experiments were performed and analyzed. Moreover, there are major differences in what was studied in each experiment. Rahimi and Juliano studied the ability of chronic NGF delivery to restore normal cortical activity in a cortex with ⬎80% AChE depletion, while we demonstrated the lack of ability of acute NGF application to induce transient plasticity in a cortex devoid of ⬎35% of its 91 • JANUARY 2004 • www.jn.org 434 N. PRAKASH, S. COHEN-CORY, S. PENSCHUCK, AND R. D. FROSTIG FIG. 8. NGF penetrates the cortex through holes or slits in the dura and arachnoid mater (durotomy). Left: NGF-IR (red arrows denote obvious penetration areas) is seen as green in this fluorescent photomicrograph of the 1st 60-m tangential section from the pial surface in the barrel cortex. Inset: the black and white image is of the whole cortex during the imaging session. The thin black ovals are the sites of the 3 dural slits; a 4th small hole was also made in this rat at the red arrow. NGF-IR through that hole is shown here. The dark black circle approximates the area at half-height border of the C2-whisker functional representation. The white arrowhead points to a reference surface vein in both the inset and the photomicrograph. Right: NGF-IR was detected under a dural slit as green in these 2 fluorescent photomicrographs of 1 40-m coronal section in the barrel cortex of a different rat. The right image shows the mediolateral extent of visible NGF-IR under a dural slit (between red arrows). The left image is a higher power image taken from the region indicated by the area outlined in white and demonstrates that in this rat NGF-IR was detectable ⬃50 m below the pial surface. Control slices not shown here had staining patterns similar to the regions outside the red arrows. AChE fibers. Finally, a recent study using a novel antibody to TrkA reported the existence of TrkA-IR on ChAT-IR neurons in layers II-III and layers V–VI in the rat visual cortex (Tropea et al. 2002). If these finding could be replicated in the somatosensory cortex of the adult rat and the specificity of this novel antibody further verified, it may help in clarifying the differences between ours and Rahimi and Juliano’s studies. In particular, if there are TrkA-IR and ChAT-IR neurons in somatosensory cortex in different cortical layers, there may be different layer-dependent effects. In Rahimi and Juliano’s study, the fibroblast grafts were placed in the deep layers of the cortex (1.1-mm depth), while our findings where occurring as NGF was penetrating layer I (see following text). There are at least two other potential ways to complement our studies to obtain a better insight for the role of the BFCS system in NGF’s ability to induce rapid plasticity of a WFR. The first method would be to stimulate the BFCS and observe whether WFR’s plasticity is identical to NGF’s induced plasticity. However, it is currently not possible to specifically stimulate only the BFCS within the BF. Nevertheless, stimulation of the BF (which includes cholinergic, GABAergic, and other components) (Dykes 1997) in the rat typically induces enhancements of neuronal activity (Jimenez-Capdeville et al. 1997; Metherate and Ashe 1991, 1993). Although it remains to be shown, such enhancements at the neuronal level could, in principle, underlie the NGF-induced plasticity of a WFR. The second way to test whether activation of the BFCS is implicated in NGF’s-induced plasticity of a WFR is to pharmacologically increase acetylcholine receptor activity. Such experiments are reported elsewhere (Penschuck et al. 2002) and used a protocol similar to the protocol reported here, but instead of NGF, cholinergic agonists such as carbachol or nicotine were topically applied to the durotomized cortex. In these experiments, both nicotine and carbachol caused a transient increase in the area of WFR similarly to NGF. However, NGF and nicotine had identical time courses for this increase, whereas carbachol had a slower time course. In addition, nicotine and NGF both augmented a WFR by increasing the height of the WFR in addition to the expansion of area, whereas carbachol only expanded the area of a WFR without the increase in height (compare augmentation to expansion in Fig. 3 for the depiction J Neurophysiol • VOL of both cases). Overall, these results are consistent with the hypothesis that activation of acetylcholine receptors could explain WFR plasticity. They also suggest that NGF’s rapid plasticity could be mediated predominantly through nicotinic receptors. Is ngf rapidly inducing plasticity by activating nicotinic receptors in cortical layer I? Previously, we showed that at the time point when the NGF-induced plasticity of a WFR was maximal, NGF penetrated layer I of the somatosensory cortex (Prakash et al. 1996b). In the current study, the more limited penetration NGF further suggests that topical application of NGF can affect evoked cortical activity by inducing acetylcholine release from BFCS projection axons within layer I. Assuming that the sensitivity for the detection of the NGF using immunohistological techniques is optimal, the correlation between the penetration and plasticity could be interpreted in the following way. NGF binding to BFCS projections within layer I causes enhancement of ACh release. Indeed layer I contains the highest laminar densities of ACh axons and varicosities originating from the BFCS (Mechawar et al. 2000), thus providing a rich substrate for NGF action. The target of the cholinergic fibers in layer I could be the apical dendrites of pyramidal and bipolar cells from layer II to V that extend to layer I (reviewed by Nieuwenhuys 1994) or direct activation of neurons located within layer I. One clue to the type of cholinergic target of NGF is its time course. NGF’s plasticity time course and its characteristics (augmentation) seem identical to nicotine’s (Penschuck et al. 2002). These findings suggest that NGF’s-induced rapid plasticity acts predominantly through activation of nicotinic cortical receptors within layer I. This suggestion is supported by recent findings that demonstrate that although nicotinic receptors can be found in all layers of the adult rat somatosensory cortex, they are heavily concentrated in layer I (Levy and Aoki 2002). Indeed, recent evidence demonstrates directly that ACh affects neuronal activity in layer I through nicotinic receptors by showing that almost all of layer I interneurons can be excited by ACh nicotinic agonists. Furthermore, by activating 91 • JANUARY 2004 • www.jn.org MECHANISMS UNDERLYING NGF CORTICAL PLASTICITY selectively the nicotinic receptors of layer I interneurons, it was demonstrated that the predominant role of these interneurons is to inhibit layer II and III nonpyramidal cells, most likely GABAergic interneurons, and thus probably cause a disinhibition of cortical networks (Christophe et al. 2002). These authors further demonstrate that some of layer I interneurons have indeed direct axonal projections to cortical layers II and III. Indirect activation of layers II and III from layer I has also been demonstrated. Stimulating layer I has been found to induce LTP in layers II/III of the cortex indirectly via the cholinergic processes that also reach layer I (Hess and Donoghue 1999). Taken together, cholinergic activation of layer I by NGF can cause large-scale cortical plasticity in deeper cortical layers through direct and indirect mechanisms that could act in concert and seem to be the result of activation of layer I nicotinic receptors. Modulatory versus trophic effects of NGF The presence of NGF in the adult cortex is thought to serve long-term processes, such as survival and maintenance of neurons, as an extension of its role during cortical development. These processes occur after NGF binds to TrkA and/or p75 (reviewed in Bothwell 1995; Kaplan and Miller 1997). NGF is rapidly internalized on binding to p75 (Kiss et al. 1993) and/or TrkA (Grimes et al. 1997; Zapf-Colby and Olefsky 1998) into a vesicle that is transported back to the cell body where its trophic actions occur via second-messenger cascades within the cell body and nucleus (recently reviewed in Sofroniew et al. 2001). However, initially on binding, and throughout the transport process, within this vesicle, the NGF/ receptor complex actively induces second-messenger pathways. Moreover, recently evidence has accumulated to suggest that the signals induced by NGF may play other roles within the cortex that result in more immediate effects such as increasing synaptic efficacy. For example, in slices of rat visual cortex NGF induced a rapid increase in the height of impulseevoked excitatory postsynaptic currents through AMPA and NMDA receptors, within 5–15 min after NGF application linking NGF action to fast modulation via glutamate release (Carmignoto et al. 1997). The authors speculate that the NGF action is mediated presynaptically through severed cholinergic nerve projections in the slice. In addition, adult cortical cell cultures showed rapid (within 10 min) activation of calciumdependent potassium channels by NGF (Holm et al. 1997). Taken together, these results illustrate that NGF is capable of inducing plasticity of adult neurons on several levels. First, initially on binding to TrkA, NGF induces acute changes in cortical activity through changes in synaptic efficacy; such changes may continue to be induced while the NGF/receptor complex is transmitted back to the cell body. Once the complex reaches the cell body, there are also induced changes in protein expression. Hence in adult rat brain, NGF can rapidly augment evoked sensory activity by modulating activity within the BFCS axons, as well as maintaining the survival of the BFCS cells. NGF-induced plasticity: a proposed model for cortical modulation of a diffuse modulatory projection system The cortex is generally portrayed as a passive recipient of modulatory systems projections including cholinergic, dopaJ Neurophysiol • VOL 435 minergic, noradrenergic, serotonergic, and histaminergic diffuse systems. Our findings implicate the cortical projections of the BFCS in the plasticity induced by NGF and therefore show that under certain conditions, the sensory cortex is not just a passive recipient of the cholinergic projections but an active participant in modulation of their activity, a modulation resulting in a large-scale plasticity of a WFR. Therefore by enhancing release of NGF, the cortex can transiently augment sensory representations like WFRs by recruiting additional neurons to respond to a stimulus, resulting in the expansion of the area of a representation, and by enhancing the strength of their responses, resulting in the increased height of the representation. It will be interesting to find out whether such cortical modulation of a diffuse modulatory system is unique to the cholinergic system or whether the cortex can also similarly modulate other diffuse modulatory systems. An interesting prediction of such a model is that the cortical outcome of NGF-induced plasticity should be different from the outcome expected from directly enhancing BF activity (i.e., a direct BF stimulation experiment). In the case of NGF-induced plasticity, the cortex modulates only on the BFCS projection fibers within the cortex. In contrast, directly stimulating the BF enhances unselectively the activity of all the different systems that reside within the BF (including the BFCS) and therefore would result in a different cortical activation pattern compared with the selective enhancement of the cholinergic projections inside the cortex itself. ACKNOWLEDGMENTS We thank J. Lee, and J. Hsu for technical assistance, Drs. R. Metherate, D. Polley, and Cynthia Chen-Bee for helpful discussions, and Genentech, Inc., for the generous gifts of NGF and NGF-antibodies. Present addresses: N. Prakash, Dept. of Neurology, University of California, Los Angeles, California 90095-6975; S. Penschuck, Neuropharmacology Dept., H. Lundbeck A/S, Ottiliavej 9, DK-2500 Valby, Denmark. GRANTS This work was supported by the fellowships from the University of California Irvine Medical Scientist Training Program and the American Heart Association (N. Prakash) as well as National Institute of Neurological Disorders and Stroke Grants NS-39760 and NS-34519 and National Science Foundation Grant IBN-9507936 (R. D. Frostig). REFERENCES Albanese A and Butcher LL. Locus ceruleus somata contain both acetylcholin esterase and norepinephrine: direct histochemical demonstration on the same tissue section. Neurosci Lett 14: 101–104, 1979. Ances BM, Buerk DG, Greenberg JH, and Detre JA. Temporal dynamics of the partial pressure of brain tissue oxygen during functional forepaw stimulation in rats. Neurosci Lett 306: 106 –110, 2001. Armstrong DM, Saper CB, Levey AI, Wainer BH, and Terry RD. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol 216: 53– 68, 1983. Baskerville KA, Schweitzer JB, and Herron P. Effects of cholinergic depletion on experience-dependent plasticity in the cortex of the rat. Neuroscience 80: 1159 –1169, 1997. Bigl V, Woolf NJ, and Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull 8: 727–749, 1982. Book AA, Wiley RG, and Schweitzer JB. Specificity of 192 IgG-saporin for NGF receptor-positive cholinergic basal forebrain neurons in the rat. Brain Research 590: 350 –355, 1992. Book AA, Wiley RG, and Schweitzer JB. 192 IgG-saporin. I. Specific lethality for cholinergic neurons in the basal forebrain of the rat. J Neuropathol Exp Neurol 53: 95–102, 1994. 91 • JANUARY 2004 • www.jn.org 436 N. PRAKASH, S. COHEN-CORY, S. PENSCHUCK, AND R. D. FROSTIG Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci 18: 223–253, 1995. Brett-Green BA, Chen-Bee CH, and Frostig RD. Comparing the functional representations of central and border whiskers in rat primary somatosensory cortex. J Neurosci 21: 9944 –9954, 2001. Bucci DJ, Holland PC, and Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J Neurosci 18: 8038 – 8046, 1998. Carmignoto G, Pizzorusso T, Tia S, and Vicini S. Brain-derived neurotrophic factor and nerve growth factor potentiate excitatory synaptic transmission in the rat visual cortex. J Physiol 498: 153–164, 1997. Chen KS, Nishimura MC, Armanini MP, Crowley C, Spencer SD, and Phillips HS. Disruption of a single allele of the nerve growth factor gene results in atrophy of basal forebrain cholinergic neurons and memory deficits. J Neurosci 17: 7288 –7296, 1997. Chen-Bee CH, Kwon MC, Masino SA, and Frostig RD. Areal extent quantification of functional representations using intrinsic signal optical imaging. J Neurosci Methods 68: 27–37, 1996. Christophe E, Roebuck A, Staiger JF, Lavery DJ, Charpak S, and Audinat E. Two types of nicotinic receptors mediate an excitation of neocortical layer I interneurons. J Neurophysiol 88: 1318 –1327, 2002. Cuello AC, Maysinger D, and Garofalo L. Trophic factor effects on cholinergic innervation in the cerebral cortex of the adult rat brain. Mol Neurobiol 6: 451– 461, 1992. Dekker AJ, Gage FH, and Thal LJ. Delayed treatment with nerve growth factor improves acquisition of a spatial task in rats with lesions of the nucleus basalis magnocellularis: evaluation of the involvement of different neurotransmitter systems. Neuroscience 48: 111–119, 1992. Dykes RW. Mechanisms controlling neuronal plasticity in somatosensory cortex. Can J Physiol Pharmacol 75: 535–545, 1997. Eckenstein F and Sofroniew MV. Identification of central cholinergic neurons containing both choline acetyltransferase and acetylcholinesterase and of central neurons containing only acetylcholinesterase. J Neurosci 3: 2286 – 2291, 1983. Fibiger HC. The organization and some projections of cholinergic neurons of the mammalian forebrain. Brain Research 257: 327–388, 1982. Frostig RD, Lieke EE, Ts’o DY, and Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci USA 87: 6082– 6086, 1990. Gage FH, Armstrong DM, Williams LR, and Varon S. Morphological response of axotomized septal neurons to nerve growth factor. J Comp Neurol 269: 147–155, 1988. Grimes ML, Beattie E, and Mobley WC. A signaling organelle containing the nerve growth factor-activated receptor tyrosine kinase, TrkA. Proc Natl Acad Sci USA 94: 9909 –9914, 1997. Gutierrez H, Miranda MI, and Bermudez-Rattoni F. Learning impairment and cholinergic deafferentation after cortical nerve growth factor deprivation. J Neurosci 17: 3796 –3803, 1997. Hefti F, Hartikka J, and Knusel B. Function of neurotrophic factors in the adult and aging brain and their possible use in the treatment of neurodegenerative diseases. Neurobiol Aging 10: 515–533, 1989. Henderson Z. Acetylcholinesterase on the dendrites of central cholinergic neurons: an electron microscopical study in the ferret. Neuroscience 28: 95–108, 1989. Hess G and Donoghue JP. Facilitation of long-term potentiation in layer II/III horizontal connections of rat motor cortex following layer I stimulation: route of effect and cholinergic contributions. Exp Brain Res 127: 279 –290, 1999. Holler T, Berse B, Cermak JM, Diebler MF, and Blusztajn JK. Differences in the developmental expression of the vesicular acetylcholine transporter and choline acetyltransferase in the rat brain. Neurosci Lett 212: 107–110, 1996. Holm NR, Christophersen P, Olesen SP, and Gammeltoft S. Activation of calcium-dependent potassium channels in mouse [correction of rat] brain neurons by neurotrophin-3 and nerve growth factor. Proc Natl Acad Sci USA 94: 1002–1006, 1997. Holtzman DM, Kilbridge J, Li Y, Cunningham ETJ, Lenn NJ, Clary DO, Reichardt LF, and Mobley WC. TrkA expression in the CNS: evidence for the existence of several novel NGF-responsive CNS neurons. J Neurosci 15: 1567–1576, 1995. Jacobs SE, Fine A and Juliano SL. Cholinergic basal forebrain transplants restore diminished metabolic activity in the somatosensory cortex of rats J Neurophysiol • VOL with acetylcholine depletion [published erratum appears in J Neurosci 14: 3, 1994]. J Neurosci 14: 697–711, 1994. Jimenez-Capdeville ME, Dykes RW, and Myasnikov AA. Differential control of cortical activity by the basal forebrain in rats: a role for both cholinergic and inhibitory influences. J Comp Neurol 381: 53– 67, 1997. Kaplan DR and Miller FD. Signal transduction by the neurotrophin receptors. Curr Opin Cell Biol 9: 213–221, 1997. Kim DS, Duong TQ, and Kim SG. High-resolution mapping of iso-orientation columns by fMRI. Nat Neurosci 3: 164 –169, 2000. Kiss J and Patel AJ. Development of the cholinergic fibers innervating the cerebral cortex of the rat. Int J Dev Neurosci 10: 153–170, 1992. Kiss J, Shooter EM and Patel AJ. A low-affinity nerve growth factor receptor antibody is internalized and retrogradely transported selectively into cholinergic neurons of the rat basal forebrain. Neuroscience 57: 297– 305, 1993. Knipper M, da Penha Berzaghi M, Blochl A, Breer H, Thoenen H, and Lindholm D. Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived neurotrophic factor in the rat hippocampus. Eur J Neurosci 6: 668 – 671, 1994a. Knipper M, Leung LS, Zhao D, and Rylett RJ. Short-term modulation of glutamatergic synapses in adult rat hippocampus by NGF. Neuroreport 5: 2433–2436, 1994b. Koliatsos VE, Nauta HJ, Clatterbuck RE, Holtzman DM, Mobley WC, and Price DL. Mouse nerve growth factor prevents degeneration of axotomized basal forebrain cholinergic neurons in the monkey. J Neurosci 10: 3801–3813, 1990. Kromer LF. Nerve growth factor treatment after brain injury prevents neuronal death. Science 235: 214 –216, 1987. Lamour Y, Dutar P, and Jobert A. Topographic organization of basal forebrain neurons projecting to the rat cerebral cortex. Neurosci Lett 34: 117–122, 1982. Lehmann J, Nagy JI, Atmadia S, and Fibiger HC. The nucleus basalis magnocellularis: the origin of a cholinergic projection to the neocortex of the rat. Neuroscience 5: 1161–1174, 1980. Levy RB and Aoki C. Alpha7 nicotinic acetylcholine receptors occur at postsynaptic densities of AMPA receptor-positive and -negative excitatory synapses in rat sensory cortex. J Neurosci 22: 5001–5015, 2002. Malonek D, Dirnagl U, Lindauer U, Yamada K, Kanno I, and Grinvald A. Vascular imprints of neuronal activity: relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proc Natl Acad Sci USA 94: 14826 –14831, 1997. Malonek D and Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science 272: 551–554, 1996. Masino SA. Quantitative comparison between functional imaging and singleunit spiking in rat somatosensory cortex. J Neurophysiol 89: 1702–1712, 2003. Masino SA and Frostig RD. Quantitative long-term imaging of the functional representation of a whisker in rat barrel cortex. Proc Natl Acad Sci USA 93: 4942– 4947, 1996. Masino SA, Kwon MC, Dory Y, and Frostig RD. Characterization of functional organization within rat barrel cortex using intrinsic signal optical imaging through a thinned skull. Proc Natl Acad Sci USA 90: 9998 –10002, 1993. McKinney M, Coyle JT, and Hedreen JC. Topographic analysis of the innervation of the rat neocortex and hippocampus by the basal forebrain cholinergic system. J Comp Neurol 217: 103–121, 1983. Mechawar N, Cozzari C, and Descarries L. Cholinergic innervation in adult rat cerebral cortex: a quantitative immunocytochemical description. J Comp Neurol 428: 305–318, 2000. Merlio JP, Ernfors P, Jaber M and Persson H. Molecular cloning of rat trkC and distribution of cells expressing messenger RNAs for members of the trk family in the rat central nervous system. Neuroscience 51: 513–532, 1992. Mesulam MM, Mufson EJ, Levey AI, and Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol 214: 170 –197, 1983. Metherate R and Ashe JH. Basal forebrain stimulation modifies auditory cortex responsiveness by an action at muscarinic receptors. Brain Res 559: 163–167, 1991. Metherate R and Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse 14: 132– 143, 1993. 91 • JANUARY 2004 • www.jn.org MECHANISMS UNDERLYING NGF CORTICAL PLASTICITY Minger SL and Davies P. Persistent innervation of the rat neocortex by basal forebrain cholinergic neurons despite the massive reduction of cortical target neurons. I. Morphometric analysis. Exp Neurol 117: 124 –138, 1992a. Minger SL and Davies P. Persistent innervation of the rat neocortex by basal forebrain cholinergic neurons despite the massive reduction of cortical target neurons. II. Neurochemical analysis. Exp Neurol 117: 139 –150, 1992b. Mizukawa K, McGeer PL, Tago H, Peng JH, McGeer EG, and Kimura H. The cholinergic system of the human hindbrain studied by choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Brain Res 379: 39 –55, 1986. Nieuwenhuys R. The neocortex. An overview of its evolutionary development, structural organization and synaptology. Anat Embryol 190: 307–337, 1994. Paxinos G and Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1986. Penschuck S, Chen-Bee CH, Prakash N, and Frostig RD. In vivo modulation of a cortical functional sensory representation shortly after topical cholinergic agent application. J Comp Neurol 452: 38 –50, 2002. Penschuck S, Prakash N, Cohen-Cory S, and Frostig RD. Interaction of NGF with the cholinergic basal forebrain system during NGF-induced rapid cortical plasticity in the rat primary somatosensory cortex. Proceedings of the 1st Göettingen Conference of the German Neuroscience Society 1999; 27th Göettingen Neurobiology Conference, Stuttgart, New York: Thieme, p. 733. Pesavento E, Margotti E, Righi M, Cattaneo A, and Domenici L. Blocking the NGF-TrkA interaction rescues the developmental loss of LTP in the rat visual cortex: role of the cholinergic system. Neuron 25: 165–175, 2000. Polley DB, Chen-Bee CH, and Frostig RD. Two directions of plasticity in the sensory-deprived adult cortex. Neuron 24: 623– 637, 1999. Prakash N, Cohen-Cory S, and Frostig RD. Differential effects of neurotrophins on the functional organization of the adult barrel cortex in vivo. Soc Neurosc Abstr 22: 997, 1996a. Prakash N, Cohen-Cory S, and Frostig RD. Rapid and opposite effects of BDNF and NGF on the functional organization of the adult cortex in vivo. Nature 381: 702–706, 1996b. Prakash N, Cohen-Cory S, and Frostig RD. NGF-induced rapid functional plasticity in the adult rat somatosensory cortex is mediated by fibers originating in the basal forebrain cholinergic system. Soc Neurosc Abstr 26: 1931, 2000a. Prakash N, Vanderhaeghen P, Cohen-Cory S, Frisen J, Flanagan JG, and Frostig RD. Malformation of the functional organization of somatosensory cortex in adult ephrin-A5 knock-out mice revealed by in vivo functional imaging. J Neurosci 20: 5841–5847, 2000b. Rahimi O and Juliano SL. Transplants of NGF-secreting fibroblasts restore stimulus-evoked activity in barrel cortex of basal-forebrain-lesioned rats. J Neurophysiol 86: 2081–2096, 2001. Rossi FM, Sala R, and Maffei L. Expression of the nerve growth factor receptors TrkA and p75NTR in the visual cortex of the rat: development and regulation by the cholinergic input. J Neurosci 22: 912–919, 2002. Sachdev RN, Lu SM, Wiley RG, and Ebner FF. Role of the basal forebrain cholinergic projection in somatosensory cortical plasticity. J Neurophysiol 79: 3216 –3228, 1998. Sala R, Viegi A, Rossi FM, Pizzorusso T, Bonanno G, Raiteri M, and Maffei L. Nerve growth factor and brain-derived neurotrophic factor increase neurotransmitter release in the rat visual cortex. Eur J Neurosci 10: 2185–2191, 1998. J Neurophysiol • VOL 437 Satoh K, Armstrong DM, and Fibiger HC. A comparison of the distribution of central cholinergic neurons as demonstrated by acetylcholinesterase pharmacohistochemistry and choline acetyltransferase immunohistochemistry. Brain Res Bull 11: 693–720, 1983. Schliebs R, Rossner S, and Bigl V. Immunolesion by 192IgG-saporin of rat basal forebrain cholinergic system: a useful tool to produce cortical cholinergic dysfunction. Prog Brain Res 109: 253–264, 1996. Sobreviela T, O’Clary D, Reichardt LF, Brandabur MM, Kordower JH, and Mufson EJ. TrkA-immunoreactive profiles in the central nervous system: colocalization with neurons containing p75 nerve growth factor receptor, choline acetyltransferase, and serotonin. J Comp Neurol 350: 587– 611, 1994. Sofroniew MV, Eckenstein F, Thoenen H, and Cuello AC. Topography of choline acetyltransferase-containing neurons in the forebrain of the rat. Neurosci Lett 33: 7–12, 1982. Sofroniew MV, Howe CL, and Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 24: 1217–1281, 2001. Tago H, Kimura H, and Maeda T. Visualization of detailed acetylcholinesterase fiber and neuron staining in rat brain by a sensitive histochemical procedure. J Histochem Cytochem 34: 1431–1438, 1986. Thompson JK, Peterson MR, and Freeman RD. Single-neuron activity and tissue oxygenation in the cerebral cortex. Science 299: 1070 –1072, 2003. Tropea D, Capsoni S, Covaceuszach S, Domenici L, and Cattaneo A. Rat visual cortical neurones express TrkA NGF receptor. Neuroreport 13: 1369 –1373, 2002. Weihe E, Tao-Cheng JH, Schafer MK, Erickson JD, and Eiden LE. Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc Natl Acad Sci USA 93: 3547–3552, 1996. Wenk GL. The nucleus basalis magnocellularis cholinergic system: one hundred years of progress. Neurobiol Learn Mem 67: 85–95, 1997. Wenk H, Bigl V, and Meyer U. Cholinergic projections from magnocellular nuclei of the basal forebrain to cortical areas in rats. Brain Res 2: 295–316, 1980. Wiley RG. Neural lesioning with ribosome-inactivating proteins: suicide transport and immunolesioning. Trends Neurosci 15: 285–290, 1992. Wiley RG. Targeting toxins to neural antigens and receptors. Semin Cancer Biol 7: 71–77, 1996. Wiley RG, Oeltmann TN, and Lappi DA. Immunolesioning: selective destruction of neurons using immunotoxin to rat NGF receptor. Brain Res 562: 149 –153, 1991. Will B and Hefti F. Behavioural and neurochemical effects of chronic intraventricular injections of nerve growth factor in adult rats with fimbria lesions. Behav Brain Res 17: 17–24, 1985. Williams LR, Varon S, Peterson GM, Wictorin K, Fischer W, Bjorklund A, and Gage FH. Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc Natl Acad Sci USA 83: 9231–9235, 1986. Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res 171: 11–28, 1979. Zapf-Colby A and Olefsky JM. Nerve growth factor processing and trafficking events following TrkA-mediated endocytosis. Endocrinology 139: 3232–3240, 1998. Zhu XO and Waite PM. Cholinergic depletion reduces plasticity of barrel field cortex. Cereb Cortex 8: 63–72, 1998. 91 • JANUARY 2004 • www.jn.org