* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Developmental biology 2008 Fates of the ectoderm: The neural tube
Binding problem wikipedia , lookup
Neuroethology wikipedia , lookup
Neuroeconomics wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Cortical cooling wikipedia , lookup
Biological neuron model wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Central pattern generator wikipedia , lookup
Synaptic gating wikipedia , lookup
Haemodynamic response wikipedia , lookup
Neural coding wikipedia , lookup
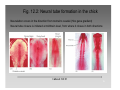
Convolutional neural network wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Neuroregeneration wikipedia , lookup
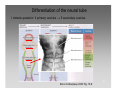
Feature detection (nervous system) wikipedia , lookup
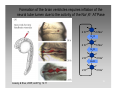
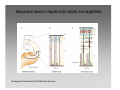
Neural oscillation wikipedia , lookup
Multielectrode array wikipedia , lookup
Molecular neuroscience wikipedia , lookup
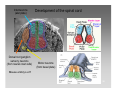
Clinical neurochemistry wikipedia , lookup
Artificial neural network wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Subventricular zone wikipedia , lookup
Types of artificial neural networks wikipedia , lookup
Neuroanatomy wikipedia , lookup
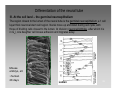
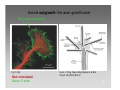
Synaptogenesis wikipedia , lookup
Recurrent neural network wikipedia , lookup
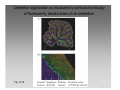
Axon guidance wikipedia , lookup
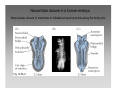
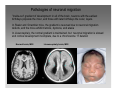
Nervous system network models wikipedia , lookup
Optogenetics wikipedia , lookup
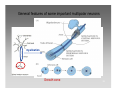
Neuropsychopharmacology wikipedia , lookup
Neural binding wikipedia , lookup
Metastability in the brain wikipedia , lookup
Neural engineering wikipedia , lookup
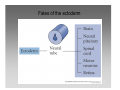
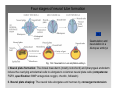
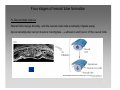
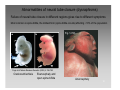
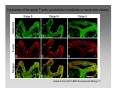
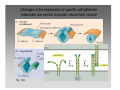
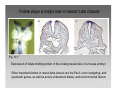
Developmental biology 2008 Fates of the ectoderm: The neural tube and brain development Lecture 1: Chapter 12 p. 373-397, 404-405 Chapter 13 p. 424-436 Stine Falsig Pedersen [email protected]/Room 527 Department of Biology University of Copenhagen Fates of the ectoderm Third lecture Second lecture First lecture Fig. 12.1 2 Fates of the ectoderm 3 Stages of neurogenesis 1. Induction of a neuron-forming region (requires competence) 2. Birth and start of migration of neurons and glial cells 3. Specification of, and commitment to, neuronal fate 4. Guidance of axon growth cones to specific targets 5. Formation of synaptic connections 6. Binding of trophic factors for survival and differentiation 7. Competitive rearrangement of functional synapses 8. Continued synaptic plasticity (Gilbert kap 13, p. 424 - after Goodman and Doe, 1993) 4 Stages of neurogenesis 1. Induction of a neuron-forming region (requires competence) 2. Birth and start of migration of neurons and glial cells 3. Specification of, and commitment to, neuronal fate 4. Guidance of axon growth cones to specific targets 5. Formation of synaptic connections 6. Binding of trophic factors for survival and differentiation 7. Competitive rearrangement of functional synapses 8. Continued synaptic plasticity (Gilbert kap 13, p. 424 - after Goodman and Doe, 1993) 5 Four stages of neural tube formation Gastrulation and neurulation in a Xenopus embryo Fig. 12.4: Neurulation in an amphibian embryo I. Neural plate formation: The dorsal mesoderm (mostly notochord) and pharyngeal endoderm induce the overlying ectodermal cells to elongate to columnar neural plate cells (competence: FGF8, specification: BMP antagonists noggin, chordin, follistatin) 6 II. Neural plate shaping: The neural tube elongates and narrows by convergent extension Four stages of neural tube formation III. Neural plate bending Medial hinge point (MHP) and dorsolateral hinge point (DLHP) cells anchor to notochord and surface ectoderm, respectively, and are induced to become wedge-shaped The actin cytoskeleton apically in the neural plate cells contracts (Factin, shroom, myosinII, MARCKs) BMPs inhibit, and the BMP antagonist noggin favors, DLHP formation; sonic hedgehog inhibits it by reducing noggin expression The presumptive ectoderm pushes inward on the neural plate cells Neural tube folding in the chick embryo 7 Four stages of neural tube formation IV. Neural plate closure Neural folds merge dorsally, and the neural crest cells eventually migrate away. Apical lamellipodial cell protrusions interdigitate → adhesion and fusion of the neural folds 8 Neural tube closure in a human embryo Neural plate closure in mammals is initiated at several points along the body axis Fig. 12.5A-C 9 Abnormalities of neural tube closure (dysraphisms) Failure of neural tube closure in different regions gives rise to different symptoms Most common is spina bifida, the mildest form (spina bifida occulta) affecting ~10% of the population Fig. 12.5D Copp et al Nature Reviews Genetics (2003) 4:784-793 Craniorachischisis Exencephaly and open spina bifida Anencephaly 10 Contraction of the apical F-actin cytoskeleton contributes to neural tube closure Zolessi & Arruti (2001) BMC Developmental Biology 1:7 11 Changes in the expression of specific cell adhesion molecules are central to proper neural tube closure Fig. 12.6 12 Folate plays a major role in neural tube closure B C Fig. 12.7 Expression of folate binding protein in the closing neural tube of a mouse embryo Other important factors in neural tube closure are the Pax3, sonic hedgehog, and openbrain genes, as well as poorly understood dietary and environmental factors 13 Primary and secondary neurulation Primary neurulation, the folding and closure of the neural tube as described above, creates the brain and most of the spinal cord. In mammals, neurulation caudal to the future upper sacral level occurs by secondary neurulation. In the tail bud, a stem-cell population that is the last bit of the retreating primitive streak, mesenchymal cells undergo condensation and epithelialization to form a tube, the lumen of which is continuous with that of the primary neural tube. Fig. 12.8 Secondary neurulation in a 25 somite chick embryo 14 Fig. 12.2: Neural tube formation in the chick Neurulation occurs in the direction from rostral to caudal (Hox gene gradient) Neural tube closure is initiated at midbrain level, from where it closes in both directions =about 33 h! 15 Fig. 12.2: Neural tube formation in the chick – overview at 24 h Neurulation occurs in the direction from rostral to caudal 16 Fig. 12.2: Neural tube formation in the chick - overview 17 Differentiation of the neural tube I. Anterior-posterior: 3 primary vesicles → 5 secondary vesicles Boron & Boulpaep 2003 Fig. 10-6 18 Formation of the brain ventricles requires inflation of the neural tube lumen due to the activity of the Na+,K+ ATPase 2 K+ 3 Na+ H2O 2 K+ 3 Na+ H2O 2 K+ 3 Na+ H2O 2 K+ Lowery & Sive, 2005; and Fig. 12.11 3 Na+ 19 Formation of the brain ventricles requires inflation of the neural tube lumen due to the activity of the Na+,K+ ATPase How do we know? The Snakehead mutant lacks functional Na+, K+ ATPases, and cannot inflate the brain ventricles! 20 Differentiation of the neural tube I. Anterior-posterior side view of developing and adult brain and spinal cord 21 Differentiation of the neural tube II. Dorsal-ventral Sonic hedgehog (shh) from the notochord induces the medial hinge point cells to become the floor plate, and TNF-β family proteins from the dorsal ectoderm induces the dorsalmost part of the neural tube to become the roof plate. The combination of ventral-to-dorsal shh gradient (ventralizing signal) and dorsal-toventral TNF-β family gradient (dorsalizing signal) induces the development of different types of neurons along the dorso-ventral axis (i.e. specification of neuronal fate depends on cell position relative to the floor plate). 22 Differentiation of the neural tube III. At the cell level – the germinal neuroepithelium The region closest to the lumen of the neural tube is the germinal neuroepithelium, a 1 cell layer thick neuronal stem cell region. Nuclei move up and down during cell cycle, with those of dividing cells closest to the lumen. At division (neuronal birthday, after which it is in Go), one daughter cell looses adhesion and migrates away. Mouse embryo, e9 ~human 28 days Lumen 23 Differentiation of the neural tube III. At the cell level – neuronal differentiation The migrated neuronal progenitor cells and glial cells derived from them create the mantle- or intermediate zone (gray matter), and myelinated neuronal axons create the marginal zone (white matter). The germinal neuroepithelium becomes the ventricular zone. The cells in the ventricular zone are largely pluripotent – their fate is specified close to or after the last mitotic division. 24 Neuronal growth and differentiation along the neural tube Spinal cord In the spinal cord, the three-zone pattern is maintained throughout development 25 Interneurons (alar plate) Dorsal root ganglion sensory neurons (from neural crest cells) Development of the spinal cord Motor neurons (from basal plate) Mouse embryo e11 26 Neuronal growth and differentiation along the neural tube Cerebellum A second germinal zone, the external granule cell layer, is formed at the outside border of the neural tube by migrating neuronal precursors. These form the granule neurons, that migrate back and form an internal granule layer. The ventricular neuronal stem cells form the Purkinje cells. Bergman glia 27 Cerebellar organization as visualized by confocal microscopy of fluorescently labeled slices of rat cerebellum Fig. 12.18 28 Neuronal growth and differentiation along the neural tube Cerebrum Neocortex is created by neuronal precursors migrating out from the mantle zone The neocortex stratifies into 6 functionally distinct layers The main type of neurons in neocortex is pyramidal neurons, and each layer contains different proportions of these and various non-pyramidal neurons Cortical layer 6 5 4 3 21 29 Neocortical neurons migrate both radially and tangentially Nadarajah & Parnavelas 2002 Nat Rev Neurosci 30 Pathologies of neuronal migration ”Inside-out” gradient of development: in all of the brain, neurons with the earliest birthdays populate the inner, and those with later birthdays the outer, layers. In Reeler and Scrambler mice, the gradient is reversed due to neuronal migration defects, and the mice exhibit tremors, dystonia, and ataxia In Lissencephaly, the normal gradient is maintained, but neuronal migration is slowed and cortical development incomplete, due to a chromosome 17 deletion Normal brain, MRI Lissencephaly brain, MRI 31 Neuronal migration, axonal outgrowth, and guidance cues ”The axon growth cone can in many ways be thought of as a neural crest cell on a leash” 32 General features of some important multipolar neurons myelination Growth cone 33 Neuronal migration and glial guidance In many regions of the CNS, neurons migrate on radial glial cell processes. Ex. Bergman glia in the cerebellum. Weaver mouse: inability of granule neurons to migrate on Bergman glia → defective neuronal migration in cerebellum. Not all neurons use radial glia to travel on– but they all need guidance cues to reach the right location. 34 Axonal outgrowth: the axon growth cone Microspikes/filopodia Fig. 12.24 Red: microtubuli Green: F-actin Huot, J. Prog. Neuro-Psychopharm. & Biol. Psych. 28 (2004) 813–8 35 Neuronal outgrowth: the axon growth cone The actin microspikes mediate neuronal pathfinding The microtubules mediate axon elongation Guidance cues direct the growth cone by modulation of actin polymerization and organization Hubert et al (2003) Ann Rev Neurosci 26:509-63 37 Forces working on the axon growth cone Laminin/integrin Netrins/DCCs + UNCs EphA7/ephrinA5 semaphorin 1/plexin Modified from Hubert et al (2003) Ann Rev Neurosci 26:509-63 NB: this is a simplified summary of some important players – whether a given signal is in fact repulsive or attractive is often cell-type specific! 38 Forces working on the axon growth cone Dorsal spinal cord explant Floor plate explant Netrins are secreted proteins, which interact with UNC or DCC receptors, and which are most frequently chemoattractants for axon growth cones Ex.: netrin-1 and netrin-2 direct commisural neurons to the ventral midline Control COS cell Dorsal spinal cord explant Netrin-1 COS cell Netrin-2 COS cell Fig. 13.22 & 13.23 39 Forces working on the axon growth cone Netrins can also act as chemorepellants The outcome depends on the netrin receptors present on the axon Ex.: trochlear nerve outgrowth is inhibited by netrin-1 Dorsal spinal cord explant Control COS cells Netrin-1 COS cells Floor plate cells 40 Forces working on the axon growth cone Laminin/integrin Netrins/DCCs + UNCs EphA7/ephrinA5 semaphorin 1/plexin Modified from Hubert et al (2003) Ann Rev Neurosci 26:509-63 Eph/ephrin semaphorin 3,5/plexin Slit/Robo 41 Forces working on the axon growth cone Ephrins are membrane proteins which interact with Eph receptors on the growth cones, and generally act as repulsive guidance cues Ephrins are expressed in the posterior part of the schlerotome Eph receptors are expressed in the motorneurons Eph/ephrin interactions inhibit motor neuron migration in the developing neural tube Rostral/ anterior Caudal/ posterior Ephrin expression Motor neurons Wang & Anderson Neuron (1997) 18(3):383-96 42 Where are we? The somites and their derivatives 43 Forces working on the axon growth cone Ephrins are membrane proteins which interact with Eph receptors on the growth cones, and generally act as repulsive guidance cues Ephrins are expressed in the posterior part of the schlerotome Eph receptors are expressed in the motorneurons Eph/ephrin interactions inhibit motor neuron migration in the developing neural tube Rostral/ anterior Caudal/ posterior Ephrin expression Motor neurons Wang & Anderson Neuron (1997) 18(3):383-96 44 Forces working on the axon growth cone Laminin/integrin Netrins/DCCs + UNCs EphA7/ephrinA5 semaphorin 1/plexin Modified from Hubert et al (2003) Ann Rev Neurosci 26:509-63 Eph/ephrin Neurotrophins: BDNF, NGF, NT-3, NT-4 semaphorin 3,5/plexin Slit/Robo 45 Forces working on the axon growth cone Neurotrophins promote survival of specific neuronal and glial populations by locally counteracting the apoptotic cell death that would occur in their absence. Survival depends on competition for a limited supply of neurotrophins. Neurotrophins also act as chemoattractants Ex.: chemoattraction: Dorsal root ganglion neuron turning in response to NT-3 Fig. 13.27 46 Forces working on the axon growth cone The specific effect of a given neurotrophic factor on axonal outgrowth differs between different types of neurons Fig. 13.29 47 Neurotrophins and neuronal survival Parkinsons disease (PD) is a neurodegenerative disease associated with loss of midbrain (substantia nigra) dopaminergic neurons. GDNF enhances survival of these neurons. Astrocytes transfected to produce GDNF and injected into the substantia nigra rescue dopaminergic neurons (stained in black in the fig.) in a mouse model (OHDA lesion) of PD. 48 Cunningham & Su (2002) Exp. Neurol.174, 230–242 Take-home points: Stages of neurogenesis, and mechanisms involved in each step Induction of a neuron-forming region (requires competence) Birth and start of migration of neurons and glial cells Specification of, and commitment to, neuronal fate Guidance of axon growth cones to specific targets Binding of trophic factors for survival and differentiation Formation of synaptic connections Competitive rearrangement of functional synapses Continued synaptic plasticity 49 A few extra slides that you might find useful 50 Eph/ephrin signaling in development Two major classes of ephrins: ephrin A, which are GPI-anchored, and ephrin B, which are transmembrane. Ephrins work via receptor tyrosine kinases, the eph receptors, to modulate growth cone F-actin organisation and polymerization. Ephrin signaling can be both forward, in the direction of the eph receptor, and reverse, in the direction of the ephrin ligand. Huot, J. Prog. Neuro-Psychopharm. & Biol. Psych. 28 (2004) 813– 818 51 Neurotrophins and neuronal survival Neurotrophins promote survival of specific neuronal and glial populations by locally counteracting apoptotic cell death. Survival depends on competition for a limited supply of neurotrophins. 52 Vecino et al (2004) Int. J. Dev. Biol. 48: 965-974 Neuronal growth and differentiation along the neural tube Time course of development of mouse cerebrum Mouse Human (approx.) 3½-4 weeks 5 weeks 8 - weeks Adult 53