* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cardiac troponin I gene knockout - University of Wisconsin–Madison
Paracrine signalling wikipedia , lookup
Gene nomenclature wikipedia , lookup
Western blot wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Proteolysis wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Gene regulatory network wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Gene expression wikipedia , lookup
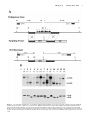
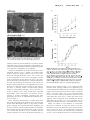
Original Contributions Cardiac Troponin I Gene Knockout A Mouse Model of Myocardial Troponin I Deficiency XuPei Huang, YeQing Pi, Kevin J. Lee, Anne S. Henkel, Ronald G. Gregg, Patricia A. Powers, Jeffery W. Walker Abstract—Troponin I is a subunit of the thin filament–associated troponin-tropomyosin complex involved in calcium regulation of skeletal and cardiac muscle contraction. We deleted the cardiac isoform of troponin I by using gene targeting in murine embryonic stem cells to determine the developmental and physiological effects of the absence of this regulatory protein. Mice lacking cardiac troponin I were born healthy, with normal heart and body weight, because a fetal troponin I isoform (identical to slow skeletal troponin I) compensated for the absence of cardiac troponin I. Compensation was only temporary, however, as 15 days after birth slow skeletal troponin I expression began a steady decline, giving rise to a troponin I deficiency. Mice died of acute heart failure on day 18, demonstrating that some form of troponin I is required for normal cardiac function and survival. Ventricular myocytes isolated from these troponin I– depleted hearts displayed shortened sarcomeres and elevated resting tension measured under relaxing conditions and had a reduced myofilament Ca sensitivity under activating conditions. The results show that (1) developmental downregulation of slow skeletal troponin I occurs even in the absence of cardiac troponin I and (2) the resultant troponin I depletion alters specific mechanical properties of myocardium and can lead to a lethal form of acute heart failure. (Circ Res. 1999;84:1-8.) Key Words: ischemia n heart failure n cardiac development C ontraction of heart muscle is triggered by calcium binding to the troponin-tropomyosin regulatory complex on cardiac muscle filaments. One protein subunit of this regulatory complex is troponin I (TnI), a 24-kDa phosphoprotein thought to play a central role in communicating calcium binding to activation of the actin filament.1–3 Understanding TnI function in the heart is important, because TnI may be modified in certain cardiac-diseased states. Selective proteolysis of TnI has been reported to underlie the pathology of stunned myocardium.4 Moreover, TnI may become depleted in ischemic,5 infarcted,6 – 8 and possibly failing myocardium.9 A cardiac-specific TnI isoform (cTnI) is released from damaged myocardial tissue, providing a clinically useful serum marker for diagnosis of some acute coronary (or ischemic) syndromes.10 Importantly, this TnI loss may not be restricted to necrotic myocardial tissue but may also occur in tissue that survives and attempts to maintain organ function.7–9 Interpretation of the effects of TnI loss in animal models of cardiac disease is often complicated by other cellular changes that occur in parallel.5– 8 On the other hand, selective removal of TnI has been reported,11,12 but this approach limits investigation to permeabilized fiber bundles. A more complete understanding of the physiological consequences of myocardial TnI depletion in vivo is needed. To better understand and control TnI expression in the heart, we knocked out the gene for cTnI in mice. Two main TnI genes are expressed in the mammalian heart under the control of a developmentally regulated program.13 Fetal TnI, which is identical to slow skeletal TnI (ssTnI), is expressed first and predominates throughout embryonic and fetal development. Around embryonic day 10, cTnI begins to be expressed. Soon after birth, cTnI accounts for roughly half of the total thin-filament TnI content, and then cTnI predominates throughout adulthood in most mammals, including humans.13–15 Since the mechanism of the fetal to adult TnI isoform switch is not well understood, we considered 2 possible outcomes of deleting the cTnI gene. The fetal ssTnI isoform could compensate, resulting in effective replacement of cTnI with ssTnI in the adult mouse heart. Alternatively, ssTnI could be downregulated as usual during the TnI isoform switch, creating a mammalian model with a myocardial TnI deficiency. Homologous recombination in embryonic stem (ES) cells was used to delete the entire cTnI gene from the mouse genome. ssTnI was found to compensate for the absence of cTnI, but only temporarily, as ssTnI was eventually downregulated by an as-yet-unidentified mechanism. This provided an opportunity to assess the effects on cardiac function of TnI loss in a system that should be relatively free of other Received July 14, 1998; accepted October 22, 1998. From the Department of Physiology, University of Wisconsin, Madison, Wis. The current affiliation for Dr Gregg is the Departments of Biochemistry and Ophthalmology, University of Louisville, Louisville, Ky. Correspondence to Dr Jeffery W. Walker, Department of Physiology, 1300 University Ave, Madison, WI 53706. E-mail [email protected] © 1999 American Heart Association, Inc. Circulation Research is available at http://www.circresaha.org 1 2 Cardiac Troponin I Knockout confounding cellular changes known to occur in cardiacdiseased states, such as altered TnI phosphorylation8,16 and defective excitation-contraction coupling. 17 The results clearly show that TnI depletion alters both resting and active mechanical properties of ventricular myocytes and causes a lethal phenotype when TnI content drops below a critical threshold. The results also show for the first time that expression of the adult cTnI isoform is not an essential component of the switch that terminates expression of the fetal ssTnI isoform in the developing heart. This mouse strain with its predictable time-dependent loss of TnI should be useful in future investigations to establish the effects of TnI depletion on cardiac function in vivo. Materials and Methods Gene Targeting A P1 clone containing the mouse cardiac TnI gene was obtained from a mouse 129 P1 genomic library (Genome Systems, St. Louis, MO). An 11-kb SalI fragment from the P1 clone was isolated, subcloned, and found to contain the entire cardiac TnI gene (Figure 1A). A 2.5-kb SalI/BamHI fragment located upstream of exon 1 and a 2.2-kb BamHI fragment located downstream of exon 8 were used as the 59 and 39 homology units, respectively. These fragments were cloned into a pNT3 vector containing a neomycin (neo) resistance gene linked to a thymidine kinase promoter flanked by multiple cloning sites. The vector also contained a herpes simplex thymidine kinase cassette for negative selection of nonrecombinant ES cell clones. The resulting targeting vector (Figure 1A) contained a unique SalI restriction site for linearization of the plasmid before electroporation into ES cells. Homologous recombination between the targeting vector and the cognate cTnI locus deleted exons 1 to 8, or the entire cTnI allele (Figure 1A). Colony selection and target clone identification were as described.18 Targeting vector (25 mg) was introduced into 53106 ES cells. Of 256 G418 and 1-(29-deoxy-29fluoro-b-D-arabinofuranosyl)-5-iodouracil–resistant clones, 96 were analyzed and 12 were found to be correctly targeted by Southern blotting with probe 1 and subsequently with probe 2. Three correctly targeted clones were microinjected into C57B1/6J blastocysts, and blastocysts were implanted in pseudopregnant 129 mice. Appropriate breeding was used to produce mice either heterozygous or homozygous for the targeted cTnI allele. Southern blot analysis was initially used for genotyping (Figure 1B). Genomic DNA was isolated from tail biopsies using Purgene DNA isolation (Gentra Systems). Five micrograms of genomic DNA was digested and then size fractionated on agarose gels, transferred to nylon membranes, and probed with DNA labeled with 32P by a random oligonucleotide protocol. Probe 1 detected a 6.5-kb EcoRI fragment from the wild-type cTnI allele and a 4.8-kb EcoRI fragment from the targeted allele (Figure 1B). A polymerase chain reaction (PCR)– based assay was also developed for rapid screening. Primers were designed to produce a 630-bp and a 390-bp fragment for the wild-type and targeted alleles, respectively. loads were standardized by a bicinchoninic acid protein assay before electrophoresis and by quantitative densitometry of Coomassie blue–stained gels as described.19 Histology Care and handling of mice were carried out according to institutional guidelines approved by the Association for Assessment and Accreditation of Laboratory Care (AAALAC) International. Mice were killed by cervical dislocation. For light microscopy, hearts were quickly excised and immersed in 10% formaldehyde solution at room temperature. Fixed hearts were sectioned into 50-mm-thick slices, stained with hematoxylin and eosin, and then viewed under a Nikon Diaphot inverted microscope equipped with a 53 objective and National Institutes of Health Image software. Lung tissue was rapidly frozen in liquid nitrogen after lungs were inflated with air to '50 mm hydrostatic pressure. Frozen tissue was sectioned into 5- to 10-mm-thick slices and viewed under the light microscope. For electron microscopy (EM) analysis, excised hearts were rapidly immersed in PBS containing 2% (vol/vol) paraformaldehyde and 2% (vol/vol) glutaraldehyde. Blocks of tissue 1 mm2 were dissected, embedded in resin, sectioned, and viewed on a Philips CM120 transmission electron microscope. Force Measurements Excised hearts were depleted of blood by massage in Ringer’s solution, and then ventricular tissue was diced and homogenized for 3 to 5 s in 5 mL of relaxing solution using a Polytron homogenizer. Cells were collected by differential centrifugation on a tabletop centrifuge and then incubated for 6 minutes at room temperature in relaxing solution containing 0.3% Triton X-100 and 0.5 mg/mL BSA. Skinned single myocytes (or small bundles of up to 4 cells) were attached to a Cambridge model 403 force transducer, and force output was monitored as described.20 A passive length-tension relationship was determined in relaxing solution containing no added calcium (nominally pCa 9, where pCa-log[Ca21]). Sarcomere length (SL) was monitored by video microscopy and varied from 1.7 to 2.7 mm in 0.2 to 0.4 –mm increments. Active tension was recorded at 2.2 mm by transferring the attached myocyte into activating solution at pCa 4.5 (32 mmol/L free Ca21) as described.20 All tension measurements were carried out at 20°C to 22°C and normalized to myocyte cross-sectional area. Solutions The composition of Ringer’s solution was as follows (in mmol/L): NaCl 118, KCl 4.8, HEPES 25 (adjusted to pH 7.4 with NaOH), KH2PO4 2, MgCl2 1.2, pyruvate 5, and glucose 11. The composition of relaxing solution was (in mmol/L): KCl 80, MgATP 4, MgCl2 7.4 (1 free Mg21), EGTA 7, creatine phosphate 11, and imidazole 25 (adjusted to pH 7.0 with KOH). Measurements of maximum active tension were performed with an activating solution of essentially the same composition as relaxing solution but containing CaCl2 (added before pH adjustment) to achieve 32 mmol/L free Ca (pCa 4.5), and KCl was adjusted to maintain a constant ionic strength of 0.18 mol/L. For intermediate Ca levels between pCa 4.5 and 9, a mixing table was used to determine the amount of relaxing and activating solution to achieve pCa values of 4.8, 5.0, 5.2, 5.5, 5.8, 6.0, and 6.5.20 Immunoblotting TnI protein levels were determined by Western blotting of ventricular tissue homogenates after electrophoresis in 12% SDS-PAGE gels and transfer onto nitrocellulose paper. An anti-TnI monoclonal antibody (clone 6F9, Advanced ImmunoChemical Inc) that recognized both mouse cTnI and ssTnI was used at a dilution of 1:1000. The identities of positive bands were confirmed by isoform-specific TnI monoclonal antibodies from the same commercial source (clone 7F4 for cTnI and clone A9 for ssTnI). ssTnI was further confirmed by comigration with an antibody-positive band (clones A9 and 6F9) in mouse soleus muscle. Antibodies on immunoblots were visualized by enhanced chemiluminescence (ECL). For quantification of TnI content, ECL bands were scanned by densitometry and compared with a range of pure cTnI on the same blot to ensure linearity. Protein Statistics Data are expressed as mean6SEM and statistically analyzed by both paired and unpaired Student’s t tests. P,0.05 was considered to be a significant difference. Data fitting was carried out by use of Marquardt’s nonlinear regression method. The quality of fits was taken to be acceptable when the SEs in the estimated pCa50 and nH values were #12% and P,0.005. Results The cTnI gene was deleted by using homologous recombination in murine ES cells. A vector containing a neomycin resistance cassette was used for positive selection of Huang et al January 8/22, 1999 Figure 1. Top, Structure and detection of cTnI before and after gene targeting. A, cTnI gene structure, restriction sites for EcoRI (RI) and SalI (S), and location of probes for Southern blots. Middle, Targeting vector with positive (NEO) and negative (thymidine kinase [TK]) selectable markers. Bottom, cTnI knockout and expected PCR and Southern blot fragments. B, Top, Southern blot using DNA probe 1 of mouse genomic DNA obtained from tail biopsies of a litter of 14-day-old pups. Genotypes are indicated under each animal number. Bottom, Western blot of ventricular samples from the same pups using a monoclonal antibody that recognizes both cTnI and ssTnI. 3 4 Cardiac Troponin I Knockout TABLE 1. Summary of Mouse Phenotypes Weight* Mortality, % Body, g Heart, mg Heart/Body, % Day 17 Day 18 Homozygous (cTnI2/2) 8.861.4 47.462.4 0.5460.03 7.8 92.2 Heterozygous (cTnI1/2) 8.162.3 42.369.7 0.5160.12 0 0 Wild type 8.461.3 44.766.8 0.5360.08 0 0 *Data are from mice on day 18 after birth and are reported as mean6SEM with n$20. targeted ES cells, and a thymidine kinase gene was used for negative selection against random insertion (Figure 1). Mice heterozygous for targeted cTnI (cTnI1/–) were indistinguishable from wild-type mice in body size, general appearance, and life span (Table 1). Heterozygote crosses showed normal fertility (60/64 pairs, 94%) with low neonatal mortality (8/448, 1.8%) and produced normal litter sizes (5 to 14 pups/litter, mean57.462.6). The genotype distribution of live neonates was 27:48:25 (wild type:cTnI1/–:cTnI–/–) as determined by Southern blots (Figure 2, top), a ratio consistent with mendelian segregation. The absence of the cTnI protein in cTnI–/– animals was confirmed by immunoblots with anti-TnI antibodies (Figure 2, bottom). Remarkably, cTnI–/– animals were also indistinguishable from wild-type animals on the basis of body weight and heart/body weight ratio measured up to 18 days postnatally (Table 1). A time course of protein expression levels showed the expected fetal to adult TnI isoform switch in wild-type mouse hearts, but the switch was delayed by '3 days in heterozygotes (cTnI1/–) (Figure 2). In cTnI–/– mice, ssTnI remained elevated for at least 10 days beyond the normal time taken for the isoform switch (Figure 2), apparently compensating for the absence of adult cTnI and accounting for the normal appearance of cTnI–/– animals. At day 15 after birth, ssTnI levels began an abrupt decline that continued over the next 3 days, reaching 3669% of its original value (Figure 2). During this decline, there was no evidence of proteolytic fragments of TnI with the antibodies used. On day 18, dyspnea (difficulty breathing) and lethargy developed, and cTnI–/– animals died within hours of the onset of these overt signs. Both the extreme nature and the consistency of the phenotype were striking, as .90% of cTnI–/– animals died on day 18 after birth, with the other 10% having died on day 17 (Table 1). Initiation of a hypertrophic growth program and other forms of remodeling including infiltration and fibrosis are common responses to cardiac insufficiency.21,22 Heart tissue sections prepared from cTnI–/– animals and stained with hematoxylin and eosin revealed neither hypertrophy nor ventricular dilation even in 18-day-old animals with advanced symptoms (not shown). Gomori’s trichrome staining for fibrosis also showed no differences between wild-type and cTnI-null hearts (not shown). Examination of ventricular tissue from 17- to 18-day-old cTnI–/– mice by EM showed few changes in muscle ultrastructure. Myofibrillar disarray, fibrosis, and macrophage infiltration were minimal. The lack of obvious indications of remodeling in cTnI–/– mice may be a characteristic of this type of myofilament defect, or more likely it is a reflection of how suddenly TnI loss occurs. Regardless of the correct explanation, these observations suggest that TnI depletion is the principal defect in cTnI–/– hearts and that secondary effects, because of tissue remodeling, contribute little to the pathology. Comparison of wildtype and null cardiac myofibrils by SDS-PAGE with silver Figure 2. Time course of the TnI isoform switch. Anti-TnI immunoblots of cardiac muscle homogenates obtained from wild-type (left panels), heterozygote (cTnI1/–) (middle panels), and cTnI–/– mice (right panels) euthanized at different times after birth. Each lane contains 20 mg of ventricular protein. Graphs show time courses of mean isoform expression levels taken from 3 separate experiments. The transition point is the estimated day on which ssTnI represented half of the total TnI. Huang et al January 8/22, 1999 5 Figure 3. EM of ventricular tissue. Images showing ultrastructure of ventricular tissue from wild-type (top) or 17-day-old cTnI–/– (bottom) animals. Arrows indicate adjacent Z-lines. staining revealed no obvious differences in protein profiles (other than TnI), but given uncertainties in this analysis, we cannot rule out the possibility that other myofilament proteins were altered as a consequence of TnI depletion. Analysis of myofibrillar SL in EM sections did reveal 1 significant change in cTnI–/– animals. SL measured from Z-line to Z-line was greatly reduced in cTnI–/– mice compared with wild-type littermates (cTnI–/– SL51.260.2 mm, n510; wild-type SL52.160.1 mm, n510) (Figure 3). Shortening of sarcomeres was not observed in cTnI–/– mice up to day 14 after birth, but developed later, in parallel with the loss of ssTnI. This shortening of sarcomeres under relaxed conditions indicates the presence of Ca-independent forces in the direction of normal active muscle contraction. The nature of this force is currently unclear, but EM images showed changes in mitochondria including a 4769% (P,0.01) increase in numbers and a trend toward larger individual mitochondria. These observations are consistent with an increase in O2/ATP consumption, suggesting that ATPdependent active forces are responsible for shortened sarcomeres in Figure 3. Direct functional measurements in isolated hearts have thus far been precluded by the small size of 17- to 18-day-old mouse hearts. Histological analysis of lung tissue revealed grossly enlarged and congested pulmonary capillaries (not shown), consistent with left ventricular failure. To determine the functional defects at the cellular level, cardiac myocytes were isolated and attached to a force transducer after the surface membrane was removed by detergent skinning.20 In Figure 4. Mechanical properties of isolated ventricular myocytes. A, Isometric force under relaxing conditions as a function of SL in cTnI–/– myocytes at day 14 ( f ) and day 17 (Œ) after birth. F, Data from 17-day-old wild-type myocytes. Data are mean6SEM. #P,0.02; *P,0.005. Solid lines are fits to f(x)5A*exp[B*(x–xo)/xo)] (Reference 34), with A50.79, B52.92, and xo51.52 mm (Œ), and A50.28, B53.45, and xo51.58 mm ( f and F). B, Ca sensitivity of active tension development. Normalized tension-pCa relationships are shown for cTnI–/– myocytes at day 14 ( f ) and day 17 (Œ) after birth. Solid lines represent fits to the Hill equation: f(x)51/{112.3*exp[nH*(pCa–pCa50)]}. myocytes from 17-day-old cTnI–/– mice, isometric tension measured under relaxing conditions was significantly elevated compared with wild-type myocytes at all SLs examined (Figure 4A). Resting tension was not elevated in 14-day-old cTnI–/– myocytes (Figure 4A), so the increased resting tension at 17 days cannot be attributed to the presence of ssTnI rather than cTnI, but it is clearly the result of TnI depletion. These steady-state force measurements directly demonstrate the presence of a force responsible for the shortened sarcomeres observed in ventricular tissue sections. Finally, the effects of TnI depletion on Ca-activated tension were examined. To assess the effects of TnI loss, tension-pCa curves were compared in myocytes from 14-dayold and 17-day-old cTnI–/– mice (Figure 4B). During this 3-day period, TnI content was reduced from its maximum level to ,40% of maximum. This loss of TnI did not greatly 6 Cardiac Troponin I Knockout TABLE 2. Summary of Active Tension in Skinned Myocytes Age, d TnI Content* Po,† mN/mm2 pCa50‡ nH‡ cTnI2/2 14 9368% ssTnI 7.460.8 5.7060.03 1.5660.20 cTnI2/2 17 3669% ssTnI 9.860.5 5.3660.03 1.4060.14 Wild type 14 .90% cTnI 8.260.9 5.1960.04 1.5660.17 Wild type 17 .90% cTnI 12.860.9 5.1060.05 1.7860.20 *For cTnI2/2 animals, ssTnI content is expressed as percentage of total TnI in wild-type age-matched littermates (mean6SEM, n55); cTnI content is 0%. cTnI content in wild-type animals was not quantified with precision because of the likelihood that very low levels of ssTnI and high levels of cTnI were not in the linear range for detection by ECL with a single exposure. †Total tension (active plus resting) measured at pCa 4.5, 22°C. Data are reported as mean6SEM (n58) for all tension measurements. ‡Obtained from fits to the Hill equation of tension-pCa data, as in Figure 4B. affect maximum tension (Po) because, although Po increased somewhat, a similar increase was observed in wild-type myocytes over this same time period (Table 2). The largest effect of TnI loss was on the pCa50 value, which decreased from pCa 5.70 to 5.36 (Figure 4B). It is not likely that developmental changes in other myofilament proteins contributed to this time-dependent change in pCa50, because shifts in the tension-pCa relationship were considerably less in wild-type myocytes (Table 2). Thus, TnI depletion is associated with a decrease in the Ca sensitivity of tension development in this system. Discussion We deleted the cardiac-specific TnI gene, cTnI, with the goal of manipulating the content or the species of TnI expressed in the mouse heart. Because there are 2 main TnI genes expressed in the mammalian heart, ssTnI and cTnI (located on different chromosomes), it was unclear how ssTnI expression would change in response to deletion of cTnI. In wild-type ventricular tissue, ssTnI and cTnI are reciprocally expressed, giving rise to a time-dependent isoform switch, as follows. ssTnI is expressed first during development and then is gradually replaced by cTnI, beginning at about embryonic day 10 and finishing around postnatal day 10.13–15 At one extreme, the ssTnI expression pattern could have been completely unaffected by the absence of cTnI and the heart would be effectively depleted of ssTnI by postnatal day 10. At the other extreme, ssTnI could have remained at a high level of expression throughout development and growth, thereby fully compensating for the absence of cTnI. What we observed was somewhere between these extremes. ssTnI initially compensated for the absence of cTnI, but beginning around day 15 after birth a steady loss of ssTnI occurred, giving rise to a condition of TnI deficiency. It is clear from the present results that TnI depletion has deleterious effects on the mechanical properties of cardiac muscle, including changes in both resting and active tension. One observed defect was elevation of resting tension, but the nature of this force in the direction of sarcomere shortening is presently unclear. TnI extraction experiments in isolated ventricular trabeculae have shown that active cross-bridges are recruited (even in the absence of Ca) as TnI is removed.11 In the present study, EM sections of cTnI-null ventricular tissue revealed proliferation and enlargement of mitochon- dria, suggesting increased ATP consumption, consistent with the possibility that the force is due to recruitment of unregulated active cross-bridges as TnI is lost. In this respect the effect of TnI depletion appears to be similar to cTnI extraction in skinned cardiac muscle.11 Another defect in TnI depleted cTnI–/– myocytes was a reduced responsiveness of the regulatory system to Ca during the development of active force. This observation is different from what has been reported in skeletal muscle following extraction of TnI,23 in which an increase in Ca sensitivity was observed. This may be an indication that cardiac and skeletal muscle are fundamentally different in this respect. TnI extraction experiments have been performed in cardiac muscle, but the effects of TnI removal per se on Ca sensitivity of tension have not been presented in detail.11,12 We consider it unlikely that developmental changes in other myofilament proteins occurring between days 14 and 17 are responsible for the observed rightward shift in the tension-pCa relationship, because there was little change in the tension-pCa curve in wild-type myocytes measured in this time window. Moreover, a candidate myofilament protein has not been identified in the literature that both undergoes a significant switch during this brief period and would be able to account for such a large desensitization of the myofilaments to Ca. The observed changes in myocyte contractility with TnI loss are important in view of the possibility that TnI is altered in certain cardiac-diseased states.4 – 8 In dog myocardium subjected to coronary occlusion, all 3 troponin subunits were degraded with TnI showing the most marked change.5 Myofilament Ca sensitivity assessed by superprecipitation of actomyosin was depressed by this treatment.5 In rat myocardium subjected to 60 minutes of complete global ischemia, both cTnI and TnT were reduced by '40% to 50%, whereas other myofilament proteins were unaffected.6 The myofilament Ca sensitivity of actomyosin ATPase activity was enhanced in this experimental system,6 but this may be due to binding of cytosolic proteins to the myofilaments.24 In pig hearts subjected to acute myocardial infarction, cTnI and TnT levels were reduced by 40% to 80% in myocardial tissue remote from the infarction zone after 2 months of remodeling.7 In rat experimental myocardial infarction, cTnI content was reduced by 53% in myocytes remaining 7 days after surgery.8 In the latter case, assessment of the mechanical properties of ventricular myocytes revealed an increase in Huang et al resting tension and a decrease in Ca sensitivity of active tension.8 However, substantial increases in phosphorylation of cTnI and TnT were also observed,8 which could contribute to the observed decrease in myofilament Ca responsiveness.3,16 In the present study, any influence of TnI phosphorylation by protein kinase A can be ruled out, because the TnI isoform present in these hearts is lacking protein kinase A sites. Also, in the present study there was no evidence of new proteins bound to the myofilaments or of secondary changes due to fibrosis or tissue remodeling, which can confound interpretation of mechanical measurements in diseased tissue. These considerations strengthen our conclusion that TnI depletion by '50% to 60% is responsible for the mechanical defects observed in cTnI–/– myocytes including as much as a 2-fold increase in resting tension and a decrease in Ca sensitivity of tension by more than 0.3 pCa units. Myocardial stunning is another disease state in which TnI may be selectively modified. Evidence has been presented in a rat model of stunned myocardium that Ca-dependent proteolysis of cTnI, but not other myofilament proteins, underlies the pathology.4 Physiological measurements in intact ventricular muscle demonstrated a decreased myofilament Ca responsiveness and altered diastolic tone after stunning.25 A reduction in myofilament Ca sensitivity has also been observed in stunned porcine ventricular myocytes.26,27 It should be recognized, however, that changes in TnI in stunned myocardium may not be analogous to those in the cTnI knockout mice. Proteolysis in stunned rat myocytes produced a polypeptide fragment of TnI4 that presumably remained associated with the myofilaments; therefore, this condition cannot be considered equivalent to TnI depletion. No proteolytic fragments of TnI were detected in the present study or in other studies reporting reduced TnI levels,7,8 but such fragments could have gone undetected by the antibodies used. A different proteolytic fragment of TnI was reported in the rat complete global ischemia model,6 but evidence has been presented that this polypeptide is unrelated to TnI.23 Establishing the mechanism of ssTnI loss in the knockout mice and the relationship of this process to cTnI proteolysis in stunned myocardium and to cTnI loss during ischemic cardiac disease must await further investigation. At this point, the cTnI knockout mouse should not be considered a model of any specific human cardiac disease. It should nevertheless contribute to our understanding of the consequences of myocardial TnI deficiency and thereby facilitate understanding of certain diseased states. Ischemic heart disease can be associated with diastolic dysfunction and depressed contractile function,7,8,21,22,28 and the results with TnI knockout myocytes are consistent with the notion that TnI modification contributes to the pathology of postischemic heart disease. Specifically, we propose, on the basis of the increase in resting tension observed here, that TnI depletion could contribute to impaired relaxation, increased myocardial stiffness, and altered ventricular filling during diastole. Moreover, the observed reduction in Ca sensitivity of active tension would be expected to depress contractility in surviving regions of the beating heart. Indeed, it seems quite possible that degradation and/or depletion of TnI occurs not only in necrotic myocardial tissue, but also in myocytes that survive and contribute to dynamic contractile function.4,7,8 January 8/22, 1999 7 Current evidence argues against the possibility that TnI depletion is a common defect in heart failure,14 –16,29 although serum TnI levels can be elevated in severe forms.9 It remains a formal possibility that TnI insufficiency either due to mutation or to altered gene expression could be a factor in some forms of idiopathic cardiomyopathy in humans. The frequency of “severe” TnI mutations in the human population remains to be established, but several point mutations in TnI have recently been shown to be associated with some forms of human hypertrophic cardiomyopathy.30 The line of mice described here may complement transgenic approaches for creating mice with interesting TnI mutations (eg, by eliminating complications arising from endogenous cTnI expression). Moreover, the striking phenotype of the cTnI-null mice, including the sudden onset and highly predictable time of death, can be exploited as a bioassay for optimizing delivery of TnI genes, TnI peptides, or peptidomimetics into myocardium with potentially broad implications for treating cardiac dysfunction. Targeted ablation of the cTnI gene has shed light on the mechanism of the TnI isoform switch by showing that the appearance of cTnI in the developmental program influences ssTnI expression but is not required for the disappearance of ssTnI from the heart. These observations suggest that cardiac expression of the ssTnI gene is “set” in the developmental program to switch off, and it does so even in the absence of the adult cTnI isoform. The time course of the TnI isoform switch was delayed in cTnI1/– heterozygotes and even further delayed in homozygous cTnI–/– mice. These observations can be rationalized by a model in which a competition exists between TnI isoforms for a fixed number of binding sites on the thin filament strand (X.P.H. and J.W.W., unpublished observations, 1998). TnI molecules that do not find a site are presumably rapidly degraded by proteolysis. This simple model provides a straightforward explanation for the apparent gene dosage effect on the time course of the TnI isoform switch in heterozygous cTnI1/– mice; a reduced amount of cTnI protein would be less effective competing with ssTnI, resulting in a delayed transition point during the isoform switch. It also offers an explanation for the apparent compensation by ssTnI. In this case, the absence of cTnI prevents it from displacing ssTnI from filament sites. Eventually, however, ssTnI expression ceases, and when normal turnover reduces the filament TnI content, a condition of TnI depletion prevails. It is remarkable in the case of these cTnI mutant mice that apparent ssTnI gene inactivation occurs regardless of the consequences to the animals in terms of survival. This lack of plasticity in fetal TnI expression in the juvenile mouse heart is reminiscent of the observation in failing hearts that the fetal TnI gene, unlike many other contractile proteins,31,32 fails to become reactivated during the end stages of heart failure.14 –16,29 Taken together, these observations indicate that ssTnI expression in the mammalian myocardium is developmentally programmed to switch off and remain off. Mechanisms responsible for this inactivation of ssTnI expression are currently unclear. It is worth noting that differences in the behavior of the thin-filament regulatory system due to the presence of ssTnI versus cTnI are apparent in the data. At 14 days after birth, when wild-type myocytes have nearly a full complement of 8 Cardiac Troponin I Knockout cTnI and null myocytes have nearly a full complement of ssTnI, a difference of 0.5 pCa units was observed in pCa50 values (Table 2). Thus, myocytes expressing primarily ssTnI displayed an enhanced Ca sensitivity compared with myocytes expressing primarily cTnI, consistent with previous reports.1,33 This supports the suggestion that TnI isoforms are responsible for a large part of the difference in the Ca responsiveness of neonatal versus adult cardiac muscle.1,33 In summary, the consequences of targeted ablation of the cTnI gene include partial compensation by altered expression of ssTnI, followed by a state of TnI depletion when ssTnI expression declines. Changes in physiological properties that develop in parallel with TnI depletion include elevated Ca-independent force, suggesting impaired cardiomyocyte relaxation, a substantial reduction in the Ca sensitivity of force development, and ultimately a lethal phenotype due to acute heart failure. Because loss of ssTnI is rapid and there is little evidence of fibrosis, infiltration, or hypertrophy, we conclude that these changes in cardiac function are the result of TnI depletion. The cTnI knockout mice represent an important model system for further investigation into the mechanisms and consequences of TnI depletion and for controlling the molecular species of TnI to elucidate its role in cardiac function and in the etiology of cardiac disease. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. Acknowledgments This work was supported by National Institutes of Health grant P01 HL-47053 (to R.G.G., P.A.P., and J.W.W.). Y.Q.P. was supported by a postdoctoral fellowship from the American Heart Association, Wisconsin Affiliate, Inc. K.J.L. was supported by a Scientist Development Grant from the American Heart Association, Inc. J.W.W. was supported by a National Institutes of Health Research Career Development Award (K04 HL-03119). The authors wish to thank Drs Gary Lyons, Matthew Wolff, Timothy Kamp, Marion Greaser, and Richard Moss for helpful discussions and comments on an earlier version of this manuscript. The technical assistance of Kara Kemnitz (PCR), Inge Sigglekow (heart tissue sections), and Dr Robert Conhaim (lung tissue sections and analysis) is gratefully acknowledged. References 1. Solaro RJ, Van Eyk J. Altered interactions among thin filament proteins modulate cardiac function. J Mol Cell Cardiol. 1996;28:217–230. 2. Solaro RJ, Moir AJG, Perry SV. Phosphorylation of troponin I and the inotropic effect of adrenaline in perfused rabbit heart. Nature. 1976;262: 615– 617. 3. Holroyde MJ, Howe E, Solaro RJ. Modification of calcium requirements for activation of cardiac myofibrillar ATPase by cyclic AMP dependent phosphorylation. Biochem Biophys Acta. 1979;586:63– 69. 4. Gao WD, Atar D, Liu Y, Perez NG, Murphy AM, Marban E. Role of troponin I proteolysis in the pathogenesis of stunned myocardium. Circ Res. 1997;80:393–399. 5. Toyo-Oka T, Ross J. Ca sensitivity change and troponin loss in cardiac natural actomyosin after coronary occlusion. Am J Physiol. 1981;240: H704 –H708. 6. Westfall MV, Solaro RJ. Alterations in myofibrillar function and protein profiles after complete global ischemia in rat hearts. Circ Res. 1992;70: 302–313. 7. Ricchuiti V, Zhang J, Apple FS. Cardiac troponin I and T alterations in hearts with severe left ventricular remodeling. Clin Chem. 1997;43:990–995. 8. Li P, Hofmann PA, Malhorta A, Cheng W, Sonnenblick EH, Meggs LG, Anversa P. Myocardial infarction alters myofilament Ca sensitivity and mechanical behavior of myocytes. Am J Physiol. 1997;272:H360 –H370. 9. Missov E, Calzolari C, Pau B. Circulating cardiac troponin I in severe congestive heart failure. Circulation. 1997;96:2953–2958. 10. Antman EM, Tanasijevic MJ, Thompson B, Schactman M, McCabe CH, Cannon CP, Fischer GA, Fung AY, Thompson C, Wybenga D, Braunwald E. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. Cardiac specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335:1342–1349. Strauss JD, Zeugner C, Van Eyk JE, Bletz C, Troschka M, Ruegg JC. Troponin replacement in permeabilized cardiac muscle: reversible extraction of troponin I by incubation with vanadate. FEBS Lett. 1992;310:229–232. Strauss JD, Van Eyk JE, Barth Z, Kluwe L, Wiesner RJ, Maeda K, Ruegg JC. Recombinant troponin I substitution and calcium responsiveness in skinned cardiac muscle. Pflügers Arch. 1996;431:853– 862. Schianffino S, Gorza L, Ausoni S. Troponin isoform switching in the developing heart and its functional consequences. Trends Cardiovasc Med. 1993;3:12–17. Hunkeler NM, Kullman J, Murphy AM. Troponin I isoform expression in human heart. Circ Res. 1991;69:1409 –1414. Sasse S, Brand NJ, Kyprianou P, Dhoot GK, Wade R, Arai M, Periasamy M, Yacoub MH, Barton JR. Troponin I gene expression during human cardiac development and in end-stage heart failure. Circ Res. 1993;72:932–938. Bodor GS, Oakley AE, Allen PD, Crimmins DL, Ladenson JH, Anderson, PA. Troponin I phosphorylation in the normal and failing adult human heart. Circulation. 1997;96:1495–1500. Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. Defective excitationcontraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800 – 806. Gregg RG, Messing A, Strube C, Beurg M, Moss R, Behan M, Sukhareva M, Haynes S, Powell JA, Coronado R, Powers PA. Absence of the b-subunits of skeletal muscle dihydropyridine receptor alters expression of the a1 subunit and eliminates excitation-contraction coupling. Proc Natl Acad Sci U S A. 1996;93:13961–13966. Huang XP, Pi YQ, Lokuta AJ, Greaser M, Walker JW. Arachidonic acid stimulates protein kinase C epsilon redistribution in heart cells. J Cell Sci. 1997;110:1625–1634. Araujo A, Walker JW. Kinetics of tension development in skinned cardiac myocytes measured by photorelease of calcium. Am J Physiol. 1994;267:H1643–H1653. Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Circulation. 1990;81:1161–1172. Colucci WS. Molecular and cellular mechanisms of myocardial failure. Am J Cardiol. 1997;80:15L–25L. Shiraishi F, Yamamoto K. The effect of partial removal of troponin I and C on the Ca sensitive ATPase activity of rabbit skeletal muscle. J Biochem. 1994;115:171–173. Barbato, R, Menabo R, Carafoli E, Schiaffino S, Di Lisa F. Binding of cytosolic proteins to myofibrils in ischemic rat hearts. Circ Res. 1996; 78:821– 828. Gao WD, Atar D, Backx PH, Marban E. Relationship between intracellular calcium and contractile force in stunned myocardium. Circ Res. 1995;76:1036 –1048. Hofmann PA, Miller WP, Moss RL. Altered calcium sensitivity of isometric tension in myocyte-sized preparations of porcine postischemic stunned myocardium. Circ Res. 1993;72:50 –56. MacDonald K, Mammen PPA, Strang KT, Moss RL, Miller WP. Isometric and dynamic contractile properties of porcine skinned cardiac myocytes after stunning. Circ Res. 1995;77:964 –972. Kusuoka H, Marban E. Cellular mechanisms of myocardial stunning. Annu Rev Physiol. 1992;54:243–246. Cummins DV, Seymour AM, Rix LK, Kellett R, Dhoot GK, Yacoud MN, Barton PJ. Troponin I, T protein expression in experimental cardiac hypertrophy. Cardioscience. 1995;6:65–70. Kimuara A, Harada H, Park E-J, Nishi H, Satoh M, Nakahashi M, Hiroi S, Sasaoka T, Ohbuchi N, Nakamura T, Koyanagi T, Hwang T-H, Choo J-A, Chung K-S, Hasegawa A, Nagai R, Okazaki O, Nakamura H, Matsuzaki M, Sakamoto T, Toshima H, Koga Y, Imaizumi T, Saszuki T. Mutations in the cardiac troponin I gene associated with hypertrophic cardiomyopathy. Nat Genet. 1997;16:379 –382. Boheler KR, Schwartz K. Gene expression in cardiac hypertrophy. Trends Cardiovasc. 1992;2:176 –182. Molkenten JD, Lu J-R, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson ER. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. Westfall MV, Rust EM, Metzger JM. Slow skeletal troponin I gene transfer, expression and myofilament incorporation enhances adult cardiac myocyte contractile function. Proc Natl Acad Sci U S A. 1997; 94:5444 –5449. Fish D, Orenstein J, Bloom S. Passive stiffness of isolated cardiac and skeletal myocytes in the hamster. Circ Res. 1984;54:267–276.