* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Receptor Transduction Mechanisms
Survey
Document related concepts
Biological neuron model wikipedia , lookup
Long-term depression wikipedia , lookup
Patch clamp wikipedia , lookup
Synaptogenesis wikipedia , lookup
Membrane potential wikipedia , lookup
Resting potential wikipedia , lookup
Neurotransmitter wikipedia , lookup
Electrophysiology wikipedia , lookup
Channelrhodopsin wikipedia , lookup
NMDA receptor wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neuromuscular junction wikipedia , lookup
End-plate potential wikipedia , lookup
G protein-gated ion channel wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Signal transduction wikipedia , lookup
Transcript
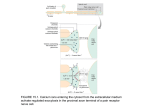
Receptor Transduction Mechanisms Secondary article Article Contents . Introduction Jenafer Evans, University of Florida, Gainesville, Florida, USA Colin Sumners, University of Florida, Gainesville, Florida, USA Craig H Gelband, University of Florida, Gainesville, Florida, USA . Opening or Closing of an Ion Channel: the Most Rapid Transduction Mechanism . Production of Either an Excitatory or Inhibitory Postsynaptic Potential by Directly Coupled Receptor/ Ion Channel Systems Neurotransmitter and peptide signalling requires receptor-mediated responses to affect the target cell. General principles of ionotropic and metabotropic receptor-mediated signalling include membrane potential alterations, calcium signalling, and kinase activity. . Initiation of Biochemical Events by Metabotropic Receptor Activation . Calcium Ions as a Major Intracellular Second Messenger . Increase in Levels of Intracellular Calcium by Activation of Both Directly Coupled Ion Channel Receptor Systems and Metabotropic Receptors Introduction . Cyclic Nucleotides as Second Messengers For cells to function together in an organism, they must have a means of communicating and coordinating growth, differentiation, metabolism, and even death. Some signal molecules, like steroids or nitric oxide, can diffuse through lipid bilayers freely. Other signal molecules depend on receptor proteins on the cell surface to transduce their message across the cell membrane. These signals, called ligands, bind specifically to their receptors with high affinity, causing a conformational change in the receptor. Agonists mimic the endogenous ligand while antagonists bind with high affinity to and prevent activation of the receptor. There are two major mechanisms by which the signal crosses the membrane once it has activated the receptor. Metabotropic receptor activation involves second messengers on the intracellular side while stimulation of ligand- Agonist . Regulation by Metabotropic Receptors of the Enzyme that Synthesizes Cyclic AMP . Regulation of Certain Ion Channels by Calcium and Cyclic Nucleotides . Transfer of Phosphate onto other Proteins by Protein Kinases: Modulation of Function . Calcium and Cyclic Nucleotide-regulated Protein Kinases . Summary gated ion channels allows passage of ions through the receptor itself (Figure 1). Opening or Closing of an Ion Channel: the Most Rapid Transduction Mechanism Ions Metabotropic receptor Ionotropic receptor Ions 2nd messenger molecules Target enzymes Cellular events Figure 1 Two distinct types of neurotransmitter receptors. Metabotropic receptor activation requires second messengers on the intracellular side. Stimulation of an ionotropic receptor allows passage of ions through the receptor itself. The resting membrane potential of excitable cells, nerve cells and muscle cells, exists due to the difference between the intracellular concentrations of the ions chloride, sodium, potassium and calcium and the extracellular concentrations of these ions. The concentration differences alone cause a concentration gradient across the membrane, while the separation of charge across the membrane causes an electrical gradient. This electrochemical gradient provides the energy necessary to drive ions across the membrane rapidly, causing action potentials, muscle contractions or intracellular signalling events, depending on the cell type. Proteins spanning the membrane allow the passage of specific ions or specific combinations of ions in response to various cellular signals including changes in the membrane potential (voltage-gated ion channels) and chemical signals (ligand-gated ion channels and cyclic nucleotide-gated channels). Opening and closing, or gating, of these ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net 1 Receptor Transduction Mechanisms channels happens rapidly, allowing for changes in membrane potential and calcium concentration, which, as we will discuss in more detail, is an important signal for various cell processes. Since ligand binding is the single event required to permit the passage of ions to initiate an action potential or muscle contraction, this signal transduction occurs more rapidly than the signals for other cell functions. Production of Either an Excitatory or Inhibitory Postsynaptic Potential by Directly Coupled Receptor/Ion Channel Systems A neurotransmitter is often classified as excitatory or inhibitory based on its action to either promote or inhibit the generation of an action potential in an excitable target cell. Stimulation of an excitatory neurotransmitter receptor generally increases the permeability of the membrane to sodium or calcium, thereby depolarizing the cell and making an action potential more likely. The resulting depolarization is called an excitatory postsynaptic potential or EPSP. In contrast, stimulation of an inhibitory neurotransmitter receptor generally increases the membrane’s permeability to potassium or chloride, thereby hyperpolarizing the cell and decreasing the likelihood of an action potential. This hyperpolarization is known as an inhibitory postsynaptic potential or IPSP. Table 1 contains a list of common neurotransmitters and the selectivity of the channels that they open. Binding of a neurotransmitter to a ligand-gated ion channel causes a conformational change in the channel, allowing particular ions to pass through the channel. Since the ion channel is actually the receptor for the ligand, this change in permeability occurs on the order of milliseconds. Synapses containing these types of ion channels are often called fast synapses, and can be excitatory or inhibitory. Nicotinic acetylcholine receptors and glutamate receptors are excitatory ligand-gated channels, while GABA (gaminobutyric acid) and glycine are inhibitory ligand-gated channels. The nicotinic acetylcholine receptor (nAChR) has been studied extensively at the neuromuscular junction and in autonomic ganglia. When acetylcholine is released from nerve terminals at the neuromuscular junction, it binds to its ionotropic receptor which undergoes a conformational change. Ionotropic receptors are ligand-gated channels which means that upon agonist binding, the receptors (which are themselves channels) open. This allows the passage of cations, mainly sodium in the case of the nAChR, into the cell, depolarizing the cell. The voltage change opens voltage-gated calcium channels and the influxing calcium binds to the contractile proteins in the muscle cell, causing a contraction. Acetylcholine also activates a second type of receptor, the muscarinic acetylcholine receptor (mAChR). Because activation of this receptor does not directly alter membrane permeability, it is referred to as a metabotropic receptor. Historically, the term metabotropic has been used to distinguish effects of neurotransmitters acting through cascade mechanisms from the effects of the same neurotransmitter to directly gate channels. For the purposes of discussion in this article, we will refer to any receptor that signals through second messengers as a metabotropic receptor. Receptors of this type will be considered in other sections of this article. Table 1 List of common neurotransmitters and their properties Neurotransmitter Ionotropic receptor Channel permeability Effect on membrane potential Cellular consequence Glutamate Acetylcholine GABA Glycine NMDA, AMPA, kainate Nicotinic GABAA Glycine Cations, Na+ >> Ca2+ Cations Anions Anions Depolarizing Depolarizing Hyperpolarizing Hyperpolarizing EPSP Muscle contraction, EPSP IPSP IPSP NMDA, N-methyl-D-aspartate; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxalone propionic acid; GABA, γ-aminobutyric acid; EPSP, excitatory postsynaptic potential; IPSP, inhibitory postsynaptic potential. 2 ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net Receptor Transduction Mechanisms When glutamate binds to its receptor on a neuron, the receptor channels open and allow sodium and calcium to pass into the cell, depolarizing the cell. If enough channels open, the cell will depolarize to threshold, the membrane potential at which the neuron fires an action potential. The depolarization will be conducted down the axon of the neuron to the nerve terminal causing calcium influx and neurotransmitter release. Thus the glutamate signal is passed from the original target neuron to another cell. Like acetylcholine, glutamate has more than one receptor type. The types of ionotropic receptors activated by glutamate are distinguished based on their affinity for glutamate structural analogues. Agonist N-methyl-d-aspartate (NMDA) binds with high affinity to NMDA receptors, while the non-NMDA receptors are named for their highaffinity agonists a-amino-3-hydroxy-5-methyl-4-isoxalone propionic acid (AMPA) and kainate. Additionally, glutamate can activate metabotropic receptors (mGluR) which have important actions on neuronal function. The binding of the inhibitory neurotransmitter GABA to the GABAA receptor produces a rapid and transient increase in membrane permeability to chloride ions. This increased chloride current hyperpolarizes the cell, pushing the resting membrane potential farther from threshold and decreasing the likelihood of an action potential. Like acetylcholine and glutamate, GABA also activates a metabotropic receptor, GABAB. Initiation of Biochemical Events by Metabotropic Receptor Activation Metabotropic receptors require second messengers to convey their signal inside the cell and stimulation results in cellular changes on the order of seconds or minutes. Such receptors reside in the plasma membrane and do not form a hydrophilic pore. Instead, they act to transduce a signal between the extracellular milieu and the cytoplasm. Specific binding of an agonist causes activation of the receptor that spans the membrane. The resulting conformational change is translated into a signal on the intracellular surface of the membrane. Some receptors, such as receptor tyrosine kinases and receptor guanylyl cyclases, have intrinsic enzymatic activity that is activated when agonist binds. Other receptors are coupled to signal transduction proteins that may have catalytic activity or may themselves activate other enzymes. In any case, the net effect is a signal that crosses the membrane. Since activation of transmembrane receptors causes a downstream effect, this opens the possibility of amplification of the signal. Stimulation of a membrane receptor can produce many active second messengers which may then affect several target proteins, thus amplifying the signal. Various neurotransmitter and hormone receptors are G protein-coupled. High-affinity binding of the neurotrans- mitter to the receptor causes a change in the receptor which allows the associated G protein a subunit to dissociate from the b and g subunits and exchange guanosine diphosphate (GDP) for guanosine triphosphate (GTP). The active GTP-bound form of the Ga subunit can then stimulate or inhibit various enzymes, depending on the particular type of subunit that typically associates with a certain receptor. Additionally, it has recently become accepted that the bg subunits act in concert and also stimulate or inhibit cellular functions. Unlike ionotropic receptors which produce responses lasting for milliseconds, metabotropic receptors can produce cellular responses that may last for seconds. In the case of metabotropic GABA and glutamate receptors, the receptors are G protein coupled to varied effector mechanisms. Through metabotropic receptors, neurotransmitters can not only be excitatory or inhibitory, but can also influence a number of other cellular functions. The GABAB receptor, for example, is coupled to two G protein types: Gi and Go. The mGluR is coupled to various signal transduction systems in different brain regions, depending on cell type. Through these transduction pathways, the metabotropic receptors can modulate cell functions like second messenger cascades, protein kinase activity and ion channel activity. Calcium Ions as a Major Intracellular Second Messenger Small changes in intracellular calcium concentration can have profound effects on the cell. The normal resting calcium concentration in a living cell is on the order of 100 nmol L 2 1. Cells maintain this low concentration by actively pumping calcium out of the cell or into organelles like the mitochondria, the sarcoplasmic reticulum or the endoplasmic reticulum. Some of the actions of calcium as a signal molecule require calmodulin. Calmodulin is ubiquitously expressed in all cell types, both as an independent molecule or as a component of an enzymatic complex. The binding of calcium to calmodulin causes a conformational change that activates or inhibits target proteins which may themselves go on to catalyse reactions. For example, when intracellular calcium concentrations are sufficiently high, calcium binds to free calmodulin molecules. This complex can then interact with and activate the calcium ATPase on the sarcoplasmic reticulum that pumps calcium into the organelle. In this way, the cell senses that the intracellular calcium levels are high and quickly sequesters the ions. Muscle cells require an increase in intracellular calcium to contract. The calcium ions bind to various contractile proteins and other enzymes which then go on to catalyse downstream reactions. In neurons, chemical synapses require calcium for the release of neurotransmitter from ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net 3 Receptor Transduction Mechanisms the presynaptic nerve terminal. Influx of calcium is necessary for the vesicles to fuse with the plasma membrane and release the neurotransmitter. Other secretory cells that secrete via vesicular release also depend on an increase in calcium current. Intracellular calcium ions can also modulate gating of ion channels. Calcium ions can increase the permeability of a certain class of potassium channels and a type of chloride channel. The voltage-gated calcium channels themselves are modulated by intracellular calcium. 1. Ligand binds to G proteincoupled receptor, coupled to Gαq αq Ca2+ Ca2+ Ca2+ VDCC GTP Because changes in calcium concentration are so crucial to many cellular processes, cells have many mechanisms by which calcium ions can enter the cytoplasm. Changes in membrane potential, as in an action potential, can cause the opening of voltage-dependent calcium channels. Calcium ions flow through the channels from the extracellular space. Agonist binding to some ligand-gated channels, such as the nicotinic acetylcholine receptors and the NMDA receptors, allows the passage of ions including calcium into the cell through the receptor itself. Agonist binding to nonchannel receptors requires second messengers to change the calcium concentration in the cell. Activation of mGluR in some cell types causes Gaq to exchange GDP for GTP. Gaq then activates a phospholipase which catalyses the conversion of phosphoinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). Excitable cells store calcium in specialized organelles called the endoplasmic reticulum (ER) or the sarcoplasmic reticulum (SR). The ER or SR sequester calcium from the cytosol by means of a calcium ATPase. These organelles have receptors for the signal molecule IP3 which is formed by stimulation of Gaqcoupled receptors. Binding of IP3 to its receptor liberates calcium from these stores. DAG, along with calcium, activates protein kinase C (PKC), a protein which adds a phosphate group to other proteins, altering their activity. Studies have shown that PKC can phosphorylate voltagegated calcium channels, allowing them to pass more calcium when they open. Thus mGluR activation causes an increase in intracellular calcium in a biphasic manner, both releasing intracellular calcium and increasing the membrane permeability to calcium (Figure 2). Other G protein cascades can also increase intracellular calcium concentrations by altering voltage-gated channels. b-Adrenergic receptor stimulation, for example, causes an increase in intracellular cAMP levels, which activates a kinase known as protein kinase A (PKA). This kinase has 4 GDP 2. Gαq exchanges GDP for GTP Ca2+ Ca2+ +++ Increase in Levels of Intracellular Calcium by Activation of Both Directly Coupled Ion Channel Receptor Systems and Metabotropic Receptors βγ 3. Gαq stimulates phospholipase to αq catalyse conversion of PIP2 to DAG + PIP2 IP3 PKC 4. DAG stimulates PKC to increase Ca2+ influx through VDCCs while IP3 binds to its receptor on the SR or ER and liberates intracellular Ca2+ stores DAG + IP3 2+ Ca Ca2+ Ca2+ Ca2+ Ca2+ 2+ Ca2+ Ca2+ 2+ Ca Ca Figure 2 Generalized Gq-coupled receptor pathway causing an increase in intracellular calcium concentration. PIP2, phosphoinositol 4,5bisphosphate; IP3, inositol 1,4,5-trisphosphate; DAG, diacylglycerol; PKC, protein kinase C; VDCC, voltage-dependent calcium channel; SR, sarcoplasmic reticulum; ER, endoplasmic reticulum; GDP, guanosine diphosphate; GTP, guanosine triphosphate. been shown to increase voltage-dependent calcium influx in the heart, skeletal and smooth muscle. Cyclic Nucleotides as Second Messengers Because cyclase enzymes are activated secondary to the binding of the ligand to the receptor, cAMP and cGMP are referred to as second messengers. Just as the level of intracellular calcium ions effects many cellular processes, the level of cyclic nucleotides can regulate many enzymatic actions and channel activity. Cyclic nucleotides can act as messengers by activating protein kinases which then go on to phosphorylate many types of proteins in the cell and alter their activity. Additionally, cAMP and cGMP can interact with target proteins directly to modify their function. Through these two mechanisms, cyclic nucleotides, like calcium, can act via direct or indirect methods. Regulation by Metabotropic Receptors of the Enzyme that Synthesizes Cyclic AMP The signal that a particular ligand passes onto a cell depends on the type of receptor for that ligand present on ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net Receptor Transduction Mechanisms the target cell as well as on the signal transduction molecules coupled to that receptor in that particular cell type. Since most cells contain thousands of types of receptors, there is a dynamic regulation of the intracellular signalling, and the signals often overlap or oppose each other. The level of cAMP in a cell is dynamically regulated by opposing G protein actions (Figure 3). Adenylate cyclase catalyses the conversion of ATP to cAMP. Gas stimulates the adenylate cyclase enzyme, while Gai inhibits it. Adrenaline binds to b-adrenergic receptors on muscle cells and the resulting signal cascade brings about glycogenolysis and changes in membrane permeability. Stimulation of the receptor liberates a Gas subunit which in turn stimulates production of cAMP via adenylate cyclase. As mentioned previously, PKA phosphorylation of calcium channels can cause increased voltage-dependent calcium influx, and such an increase in calcium current is observed upon adrenaline stimulation of the heart. The increased intracellular calcium results in the quickening of the heartbeat and increased force of contraction associated with adrenaline. Vagus nerve terminals providing sympathetic innervation to the heart release acetylcholine. The cardiac muscle expresses muscarinic acetylcholine receptors (mAChR) which are G protein coupled. The receptor is linked to both Gi and Gq so it inhibits adenylate cyclase and activates the phospholipase cascade. The Gi inhibits the cAMP production thus reducing the activity of PKA and therefore the increase in calcium influx associated with the adrenaline stimulation. Additionally, stimulation of mAChR is associated with enhanced inwardly rectifying potassium current. Interestingly, the bg subunits have been shown to interact directly with the associated potassium channel (KAch). Due to the presence of both mACh receptors and adrenaline receptors on cardiac muscle, the contractile force and rate of the heart can be dynamically controlled. Regulation of Certain Ion Channels by Calcium and Cyclic Nucleotides Membrane depolarizations can cause an increase in intracellular calcium concentration as described above. During an action potential, for example, calcium enters the cell during the depolarizing phase. To terminate the action potential, several types of potassium channels are activated to extrude potassium and return the cell to its hyperpolarized resting membrane potential. One of these channels is the calcium-activated potassium channel. There are two subtypes of this channel, the big K 1 (BK 1 ) and the small K 1 (SK 1 ). They are named for their conductances of greater than 100 pS and less than 80 pS, respectively (reviewed in Latorre et al., 1989). Many nonexcitable cells as well as cells that have action potentials express channels that are activated by binding of cyclic nucleotides (cAMP and cGMP). These channels are nonspecific cation channels which permit the flow of sodium, potassium or calcium. Sensory cells make use of the cyclic nucleotide-gated nonspecific channels. Phototransduction for example, requires cGMP-gated channels. In rods and cones, cGMP is at relatively high concentration until light stimuli activate phosphodiesterases that convert cGMP back to 5’-GMP. cGMP-gated channels close under these conditions, preventing the secretion of glutamate onto the bipolar cells of the retina. In olfaction, a specialized G protein, Golf, is stimulated to exchange GDP for GTP when the receptor is stimulated by an odorant molecule. The activated Golf then stimulates adenylate cyclase to produce cAMP which in turn opens a cation channel. Additionally, calcium can activate chloride channels. The physiological relevance of the calcium-activated chloride channel is unclear, but it probably serves to stabilize the resting membrane potential, since chloride is passively distributed across the membrane. In vascular smooth muscle, this channel causes membrane depolarization upon activation by an agonist. β–Ad mAChR βγ αs αi βγ K+ K+ K+ ++ Ca2+ Ca2+ Ca2+ Ca 2+ Ca Ca2+ VDCC –– ++ 2+ Adenylyl cyclase Ca2+ + K K+ K+ KAch K+ ++ cAMP PKA Figure 3 Crosstalk between receptor transduction pathways allows for dynamic regulation of cellular processes. b-Ad, b-adrenergic receptor; mAChR, muscarinic acetylcholine receptor; PKA, protein kinase A; VDCC, voltage-dependent calcium channel; KAch, potassium channel; cAMP, cyclic adenosine monophosphate. ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net 5 Receptor Transduction Mechanisms Transfer of Phosphate onto other Proteins by Protein Kinases: Modulation of Function Protein phosphorylation is well documented as a major mechanism for altering the activity of enzymes and channel proteins. The terminal phosphate of ATP is transferred and covalently bound to a hydroxyl group of the target protein by a kinase. Two major groups of kinases are known: tyrosine kinases and serine/threonine kinases. These groups are named for the residue they typically phosphorylate on a target protein. A phosphorylation event can be stimulatory or inhibitory, depending on the target protein and the phosphorylation site. The ultimate effect of many signal transduction cascades is to alter the phosphorylation state of target proteins. Phosphorylation events have been linked to alterations in membrane permeability, activation of gene transcription, modification of enzyme activity and more. The substrates for phosphorylation vary from cell type to cell type and the substrates present will determine the ultimate effect of the kinase. Calcium and Cyclic Nucleotideregulated Protein Kinases As mentioned earlier, DAG activates PKC isozymes which are serine/threonine kinases. Some isoforms of PKC also require an increase in intracellular calcium to be fully active. A large number of proteins have been shown to be phosphorylated by PKC, both in vitro and in vivo. Proteins containing the PKC consensus phosphorylation motif are likely targets for regulation by signal cascades activating PKC. There are subtle differences in the motifs preferred by different PKC isozymes, but all require basic residues surrounding the threonine or serine (Nishikawa et al., 1997). In the brain, PKC activation has been shown to alter ionic currents. The effects of PKC on specific ionic currents, whether stimulatory or inhibitory, are not easily generalized and depend on the presence of channel accessory subunits and the colocalization of the components of the signal cascade. PKA is the primary effector of cAMP. Another serine/ threonine kinase, PKA, phosphorylates residues that are flanked on their N-terminal side by two or more basic amino acids. PKA is thought to account for all of the effects of cAMP except for those in some central neurons including olfactory neurons, where cAMP gates an ion channel directly. cAMP-dependent phosphorylation was first demonstrated in skeletal muscle cells. Adrenaline stimulates the b-adrenergic receptor in these cells and the resulting metabolic cascade involves PKA. The increase in cAMP concentration in the cell promotes activity of PKA 6 which phosphorylates glycogen synthase, reducing the activity of the enzyme and thus reducing storage of glucose as glycogen. Additionally, PKA adds a phosphate group to glycogen phosphorylase kinase which then phosphorylates glycogen phosphorylase. The activation of this enzyme by addition of a phosphate catalyses the release of glucose from glycogen stores. PKA can also work directly by altering ion channels through phosphorylation of the channel proteins, as mentioned for the calcium channels in cardiac cells. cGMP binds to protein kinase G (PKG) which can then phosphorylate a number of target proteins. The atrium of the heart, under conditions of increased blood pressure, releases atrial natriuretic peptide (ANP). ANP stimulates sodium secretion from the kidney and relaxation of the vasculature, both effects which lower blood pressure. The receptor for ANP is a transmembrane receptor with an intracellular guanylyl cyclase domain. Binding of ANP activates the cyclase which converts GTP to cGMP which then activates PKG. Substrates for PKG include ion channels such as voltage-dependent calcium channels as well as cytoskeletal elements and nitric oxide synthase. Like PKC and PKA, PKG is a serine/threonine kinase. Few substrates of PKG are exclusively phosphorylated by PKG (reviewed in Wang and Robinson, 1997). There is an overlap with PKA specificity, so a specific phosphorylation motif is unclear. Important mediators of calcium activity in cells are the calcium/calmodulin-dependent protein kinases (CaM kinases). Some of these kinases, like myosin light-chain kinase which provokes smooth muscle cell contraction, have very specific substrates. Others, like CaM kinase II, are utilitarian in that their specific actions are determined by which substrates are present in the cell. Summary Peptide signals which cannot cross the membrane are received and transmitted to the intracellular space via two mechanisms: direct opening of ion channels or production of a receptor-mediated signal cascade. The actions of agents which open the ligand-gated ion channels are immediate because the receptor itself allows passage of ions into or out of the cell. Ligands that stimulate metabotropic receptors, however, signal through activation of intracellular proteins which produce second messengers. Increases in intracellular calcium, cAMP and cGMP are important catalysts for intracellular processes both directly and through activation of kinases. The effect a particular signal has on a target cell depends on the other active signals as well as the signal transduction processes and substrates expressed in that particular cell type. Many of these pathways interact with or oppose each other. Such crosstalk allows for finely tuned dynamic ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net Receptor Transduction Mechanisms regulation of cellular processes and integration of the many signals a cell receives from its environment. References Latorre R, Oberhauser A, Labarca P and Alvarez O (1989) Varieties of calcium-activated potassium channels. Annual Review of Physiology. 51: 385–399. Nishikawa K, Toker A, Johannes FJ, Songyang Z and Cantley LC (1997) Determination of the specific substrate sequence motifs of protein kinase C isozymes. Journal of Biological Chemistry 272: 952– 960. Wang X and Robinson PJ (1997) Cyclic GMP-dependent protein kinase and cellular signalling in the nervous system. Journal of Neurochemistry 68: 443–456. Further Reading Alberts B, Bray D, Lewis J, Raff M, Roberts K and Watson J (1994) Molecular Biology of the Cell, 3rd edn. New York: Garland Publishing. Gilman AG (1987) G proteins: transducers of receptor-generated signals. Annual Review of Biochemistry 56: 615–649. Hammond C (ed.) (1996) Cellular and Molecular Neurobiology. New York: Academic Press. Hille B (1992) Ionic Channels of Excitable Membranes, 2nd edn. Sunderland, MA: Sinauer Associates. Neer EJ and Clapham DE (1988) Roles of G protein subunits in transmembrane signalling. Nature 333: 129–134. Nicoll RA (1988) The coupling of neurotransmitter receptors to ion channels in the brain. Science 241: 545–551. Nicoll RA, Malenka RC and Kauer JA (1990) Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiological Reviews 70: 513–565. Ross EM (1989) Signal sorting and amplification through G proteincoupled receptors. Neuron 3: 141–152. Sleight RG and Lieberman MA (1998) Signal transduction. In: Sperelakis N (ed.) Cell Physiology Source Book, pp. 119–131. New York: Academic Press. Sutherland E (1972) Studies on the mechanism of hormone action. Science 177: 401–408. ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net 7