* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Polyacrylamide gels
Cre-Lox recombination wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Endomembrane system wikipedia , lookup
Molecular evolution wikipedia , lookup
Magnesium transporter wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Silencer (genetics) wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Gene expression wikipedia , lookup
Biochemistry wikipedia , lookup
Circular dichroism wikipedia , lookup
Signal transduction wikipedia , lookup
List of types of proteins wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Interactome wikipedia , lookup
Protein moonlighting wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Protein adsorption wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Community fingerprinting wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Agarose gel electrophoresis wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
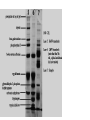
Western blot wikipedia , lookup
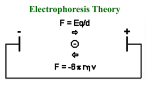
Polyacrylamide gel electrophoresis (PAGE) Electrophoresis in a polyacrylamide matrix separating or resolving molecules in a mixture under the influence of an applied electric field •PAGE used for proteins and small pieces of DNA •Similar idea to separation of DNA in agarose •2 major determinants of particle mobility are charge/mass ratio and structure •Polyacrylamide has smaller pore size- better resolution (2bp differences in DNA) and separates smaller molecules (50-500bp) •One bp has molecular mass of 660 Daltons (Dalton=gm/mole) •1 kb piece of DNA = 660kDa •Average protein has mass of ~ 60kDa. •PAGE used for proteins and small pieces of DNA •Acylamide is synthesized (not natural like agarose) • A typical setup consists of a gel slab sandwiched between two glass plates, with the ends enclosed in upper and lower reservoirs of buffer • Samples to be run are loaded in wells at the top of the gel, in conjunction with tracking dye. An electrical voltage is applied between the upper and lower reservoirs, causing the samples to migrate down through the gel. • Once a gel has been 'run', it is treated to reveal the positions of the samples Staining • Coomassie blue-sensitive to 0.1ug of protein • Silver- sensitive to 0.002ug of protein, based on ppt of silver ions producing brown stain, laborious. • greater sensitivity, radioactive samples can be used, allowing for exposure over time to produce images on photographic film, as seen in the sequencing gel on the right • To calibrate the relative migrations of molecules of different size, a marker lane is often added, where samples of known size will migrate to reference positions 4 components of gel 1. 2. 3. 4. Acrylamide APS TEMED Bisacrylamide • • Acrylamide forms long polymer chains Polymerization induced by APS (ammonium persulphate) which generates free radicals (charged oxygens) TEMED is a free radical stabilizer (N’N’N’N’-tetra methylene diamine) Air inhibits polymerization as it scavenges free radicals Bis acrylamide is a cross linking agent and links long polymers of acrylamide (N, N’-methylene bisacrylamide) Pore size is determined by % acrylamide and the amount of cross linker The copolymerization of acrylamide with methylenebisacrylamide produces a mesh-like network in three dimensions, consisting of acrylamide chains with interconnections formed from the methylenebisacrylamide • • • • • SDS PAGE • SDS is used in the gel mix. • SDS is –ve charged and binds to proteins, it denatures (unfolds) proteins and gives a net negative charge. • Proteins will then migrate to the anode • Proteins all have same charge to mass ratio • Can be separated based on size alone Native PAGE • No denaturing agents • Proteins separated based on size charge and shape. • Used when want to keep protein active to study conformation, self-association or aggregation, and the binding of other proteins Discontinuous versus Continuous 1. Discontinuous – Common – Better resolution – Stacker-used to stack proteins into thin band so have same starting position when enter separating gel. Proteins gel squished between “leading” and “trailing” ions in the gels make with different buffers – Resolving gel- higher % used to separate proteins, a pH change makes ions from the stacking gel move past the proteins and the proteins are no longer squished. 2. Continuous • no stacker, poor resolution, big blobs • easier to pour • Faster to run Potential problems with Polyacrylamide gels – – – – – Underloaded (bands invisible) Sloppy loading or to little concentration of protein Bent bands Tearing frowning • Cleanliness is next to godliness when making gels 1. Over loaded 3. Frowning (run too hot) 4. Bent bands. Tearing 2. Tearing 5. Sloppy loading or too low conc.