* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Protein Sulfenylation in Mitochondria: Biochemistry and
Catalytic triad wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Gene expression wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Biochemical cascade wikipedia , lookup
Biochemistry wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Paracrine signalling wikipedia , lookup
Expression vector wikipedia , lookup
Magnesium transporter wikipedia , lookup
Signal transduction wikipedia , lookup
Protein structure prediction wikipedia , lookup
Interactome wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Mitochondrial replacement therapy wikipedia , lookup
Protein purification wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Mitochondrion wikipedia , lookup
Western blot wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
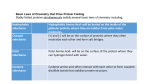
Protein Sulfenylation in Mitochondria: Biochemistry and Physiological Relevance Johannes Herrmann, Kaiserslautern Summary Mitochondria contain two aqueous compartments: the matrix and the intermembrane space (IMS). Presumably due to its evolutionary descent from the bacterial periplasm, many of the 50 to 100 proteins of the IMS reach their native structure by an oxidative folding process. The formation of disulfide bonds in IMS proteins is enzymatically catalyzed by the sulfhydryl oxidase Erv1 and the oxidoreductase Mia40. Interestingly, many proteins of the matrix and the IMS contain conserved cysteine residues which are not involved in the formation of structural disulfide bonds. Due to oxidative stress generated by the respiratory chain, these residues are prone to overoxidation which can convert thiol groups to sulfenic acid. It was recently shown that peroxide-dependent sulfenylation can serve as a molecular switch to control protein activity in a redox-dependent manner. The degree to which proteins are sulfenylated in vivo is presumably regulated by specific factors. We will use the yeast Saccharomyces cerevisiae as a model system to address the following questions: (1) Which proteins in the IMS and the matrix of mitochondria contain sulfenylated cysteine residues? (2) Under which conditions are proteins sulfenylated? (3) Which mitochondrial redox enzymes control protein sulfenylation? (4) What is the physiological relevance of these sulfenylation reactions? State of the art and preliminary work Fig. 1: Overview on cysteine oxidation processes in different cellular compartments. Oxidative protein folding is largely restricted to the periplasm, the ER and the IMS. In other cellular compartments, cysteine oxidation is found under oxidizing conditions and can serve a regulatory role. Although several examples of such thiol switches were studied in the past, it still needs to be explored how many regulatory thiols are physiologically relevant and how their redox state is regulated in vivo. For details see Herrmann and Dick, 2012; Riemer et al., 2009. Thiol oxidation serves different purposes in the cell Cysteine residues differ from all other amino acid residues in terms of their much higher chemical reactivity. Even under physiological conditions many different modifications of protein thiols are found which often are critical for the activity or the regulation of proteins. To avoid unwanted protein modifications cysteines are less frequent in proteins than all other amino acids. If present, they are very often evolutionary conserved pointing at a functional relevance of the cysteine at these positions. Cysteine oxidation processes serve many different purposes but in general two different functional roles can be distinguished (Fig. 1). In some cellular compartments such as in the bacterial periplasm, the endoplasmic reticulum (ER) and the intermembrane space of mitochondria (IMS), dedicated machineries exist which introduce stable disulfide bonds into newly synthesized proteins. In this case, thiol oxidation can significantly contribute to the resistance of proteins against thermal denaturation or proteolytic degradation (Riemer et al., 2009). However, in most cellular compartments, including the cytosol, the nucleus or the mitochondrial matrix, reducing enzymes prevent or counteract the oxidation of thiol residues so that at least under non-stressed conditions most protein thiols are reduced (Grant, 2001; Hansen et al., 2009; Kumar et al., 2011). Nevertheless, thiol oxidation can be used here in order to sense environmental conditions such as the concentrations of oxygen or hydrogen peroxide. For example, in Saccharomyces cerevisiae cysteine residues that serve as thiol switches were found in the transcription factor Yap1 which induces the expression of its target genes upon oxidative stress conditions (Delaunay et al., 2002). Thiol oxidation in the IMS of mitochondria Mitochondria contain two aqueous subcompartments, the matrix and the IMS. Even in a simple organism like baker’s yeast, more than 50 different IMS proteins were identified so far and the list of IMS protein is rapidly growing (Herrmann and Riemer, 2010). IMS proteins exhibit many functions like the transport of metabolites, metal ions, proteins and lipids between both mitochondrial membranes, the biogenesis of respiratory chain complexes or the regulation of redox processes. In animals, several pro-apoptotic factors reside in the IMS and are released when the suicide program is triggered. In several aspects, the IMS serves as an interface that mediates the exchange of molecules and the communication between mitochondria and other compartments of a eukaryotic cell. Many IMS proteins are of low molecular mass (less than 20 kDa) and contain conserved cysteine residues which are important for their translocation into mitochondria. These cysteines are oxidized during protein import and form structural disulfide bonds. Protein oxidation is mediated by a dedicated machinery, called mitochondrial intermembrane space assembly (MIA) pathway or the mitochondrial disulfide relay (Banci et al., 2009; Bien et al., 2010; Chacinska et al., 2004; Mesecke et al., 2005; Naoe et al., 2004; Terziyska et al., 2005; Weckbecker et al., 2012). The characterization of the mechanistic principles of this import machinery was a main research interest of our group during the last years. All IMS proteins are nuclear encoded and imported into mitochondria from the cytosol. The import of many of these proteins relies on the IMS protein Mia40 which directly interacts with incoming polypeptides via a hydrophobic substrate binding pocket (Banci et al., 2009; Kawano et al., 2009). By use of a redox-active CPC motif Mia40 introduces disulfide bonds into its substrates and is crucial for their stable folding (Mesecke et al., 2005). However, at least for some IMS proteins Mia40-dependent folding even occurs if the cysteine residues are removed suggesting that Mia40 exhibits a chaperone-like activity which is independent of its role as oxidoreductase (Banci et al., 2010; Weckbecker et al., 2012). Efficient oxidation of Mia40 relies on the sulfhydryl oxidase Erv1 (Allen et al., 2005; Fass, 2008; Lee et al., 2000; Mesecke et al., 2005; Tienson et al., 2009) and on cytochrome c and cytochrome c oxidase (Bihlmaier et al., 2007; Dabir et al., 2007; Farrell and Thorpe, 2005). However, not all cysteine residues of IMS proteins are oxidized. For examples c-type cytochromes contain cysteine residues that need to be reduced in order to bind heme cofactors. Moreover, several copper-binding factors reside in the IMS which rely on reduced cysteines to bind metal ions. Little is known about whether these cysteine residues are not recognized by Mia40 and therefore remain reduced or whether their redox state is controlled by reducing enzymes such as thioredoxins or glutaredoxins. Thiol oxidation in the matrix of mitochondria In inner membrane of mitochondria separates the glutathione pool of the cytosol/IMS from that of the matrix (Kojer et al., 2012). Glutathione is transported into mitochondria by a dedicated carrier protein (Roland Lill, personal communication). The matrix contains a number of enzymes to control the redox state of protein thiols, in particular glutathione reductase and thioredoxins/thioredoxin reductase which play a redundant role in cysteine reduction (Toledano et al., 2013). As a consequence, as far as we know, most cysteine residues of matrix proteins are reduced at steady state. Nevertheless, recent studies suggest that a number of protein thiols are oxidized to some degree presumably as cause of the production of oxygen radicals and hydrogen peroxide by the respiratory chain (Brandes et al., 2009). However, in the case of matrix proteins only little is known on the physiological relevance of cysteine oxidation. Redox-regulation was shown for the human branched chain aminotransferase which, upon exposure to hydrogen peroxide, is inactivated by formation of a disulfide bond between cysteines 315 an 318 (Conway et al., 2004). Protein inactivation is mediated by overoxidation of cysteine 315 which leads to a sulfonic acid via a sulfenic acid intermediate. Cysteine 318 acts as “resolving cysteine” allowing for reversible formation of a disulfide bond. The aim of this project is to identify sulfenylated cysteine residues on proteins of the matrix and the IMS of yeast mitochondria. The physiological consequences of these oxidation processes will be studied using a combination of yeast genetics and protein biochemistry and, finally, the components and conditions shall be identified which trigger these thiol switches to move them between “ON” and “OFF” states. 1.1 Project-related publications 1.1.1 Articles published by outlets with scientific quality assurance, book publications, and works accepted for publication but not yet published (max. 6) 1. Bode M, Longen S, Morgan B, Peleh V, Dick TP, Bihlmaier K, Herrmann JM. 2013. Inaccurately assembled cytochrome c oxidase can lead to oxidative stress-induced growth arrest. Antioxid Redox Signal. 18, 1597-61 2. Weckbecker D, Longen S, Riemer J, Herrmann JM. 2012. Atp23 biogenesis reveals a chaperone-like folding activity of Mia40 in the IMS of mitochondria. EMBO J. 31, 4348-4358 3. Bien M, Longen S, Wagener N, Chwalla I, Herrmann JM, Riemer J. 2010. Mitochondrial disulfide bond formation is driven by intersubunit electron transfer in Erv1 and proofread by glutathione. Mol Cell 37, 516-528 4. Bihlmaier K, Mesecke N, Terziyska N, Bien M, Hell K and Herrmann JM. 2007. The disulfide relay system of mitochondria is connected to the respiratory chain. J. Cell Biol. 179, 389-395 5. Mesecke N, Spang A, Deponte M and Herrmann JM. 2008. A Novel Group of Glutaredoxins in the cis-Golgi Critical for Oxidative Stress Resistance. Mol Biol Cell. 19, 2673-2680 6. Mesecke N, Terziyska N, Kozany C, Baumann F, Neupert W, Hell K and Herrmann JM. 2005. A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell 121, 1059-1069 A complete list of publications of the applicant can be found at: http://www.bio.uni-kl.de/zellbiologie/johannes-m-herrmann/agh-herrmann-public/ Objectives The main focus of this project is the detection of overoxidized thiols on mitochondrial proteins of baker’s yeast. Thereby four thematically related groups of questions will be addressed: 1. Which proteins contain sulfenylated thiols, i.e. potential thiol switches? Which cysteine residues are overoxidized? Which chemical modifications are present at the respective cysteine residues at which conditions? 2. Which conditions lead to sulfenylation of mitochondrial proteins? What is the influence of respiration? Is it possible to distinguish respiration-active (oxidizing) from biosynthesisactive (reductive) growth phases in respect to the sulfenylation patterns? 3. Which factors prevent the accumulation of proteins with overoxidized thiols? Which components of the quality control systems of the IMS and the matrix degrade overoxidized proteins? How are these proteins recognized? What is the relevance of glutaredoxins and the glutathion redox conditions in both mitochondrial subcompartments? 4. What is the physiological relevance of protein sulfenylation? Under which conditions are thiol switches used and to which purpose? Is triggering of these switches reversible and what is the dynamic range for these processes? 3. Bibliography Publications of the applicant are indicated with an asterisk (*) Allen, S., Balabanidou, V., Sideris, D.P., Lisowsky, T., and Tokatlidis, K. (2005). Erv1 mediates the Mia40-dependent protein import pathway and provides a functional link to the respiratory chain by shuttling electrons to cytochrome c. J Mol Biol 353, 937-944. Banci, L., Bertini, I., Cefaro, C., Cenacchi, L., Ciofi-Baffoni, S., Felli, I.C., Gallo, A., Gonnelli, L., Luchinat, E., Sideris, D., et al. (2010). Molecular chaperone function of Mia40 triggers consecutive induced folding steps of the substrate in mitochondrial protein import. Proc Natl Acad Sci USA 107, 20190-20195. Banci, L., Bertini, I., Cefaro, C., Ciofi-Baffoni, S., Gallo, A., Martinelli, M., Sideris, D.P., Katrakili, N., and Tokatlidis, K. (2009). Mia40 is an oxidoreductase that catalyzes oxidative protein folding in mitochondria. Nat Struct Mol Biol 16, 198-206. *Bien, M., Longen, S., Wagener, N., Chwalla, I., Herrmann, J.M., and Riemer, J. (2010). Mitochondrial disulfide bond formation is driven by intersubunit electron transfer in Erv1 and proof read by glutathione. Mol Cell 37, 516-528. *Bihlmaier, K., Mesecke, N., Terzyiska, N., Bien, M., Hell, K., and Herrmann, J.M. (2007). The disulfide relay system of mitochondria is connected to the respiratory chain. J Cell Biol 179, 389-395. Brandes, N., Schmitt, S., and Jakob, U. (2009). Thiol-based redox switches in eukaryotic proteins. Antioxid Redox Signal 11, 997-1014. Chacinska, A., Pfannschmidt, S., Wiedemann, N., Kozjak, V., Sanjuan Szklarz, L.K., Schulze-Specking, A., Truscott, K.N., Guiard, B., Meisinger, C., and Pfanner, N. (2004). Essential role of Mia40 in import and assembly of mitochondrial intermembrane space proteins. EMBO J 23, 3735-3746. Conway, M.E., Poole, L.B., and Hutson, S.M. (2004). Roles for cysteine residues in the regulatory CXXC motif of human mitochondrial branched chain aminotransferase enzyme. Biochemistry 43, 7356-7364. Dabir, D.V., Leverich, E.P., Kim, S.K., Tsai, F.D., Hirasawa, M., Knaff, D.B., and Koehler, C.M. (2007). A role for cytochrome c and cytochrome c peroxidase in electron shuttling from Erv1. EMBO J 26, 4801-4811. Delaunay, A., Pflieger, D., Barrault, M.B., Vinh, J., and Toledano, M.B. (2002). A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell 111, 471-481. Depuydt, M., Leonard, S.E., Vertommen, D., Denoncin, K., Morsomme, P., Wahni, K., Messens, J., Carroll, K.S., and Collet, J.F. (2009). A periplasmic reducing system protects single cysteine residues from oxidation. Science 326, 1109-1111. Farrell, S.R., and Thorpe, C. (2005). Augmenter of liver regeneration: a flavin-dependent sulfhydryl oxidase with cytochrome c reductase activity. Biochemistry 44, 1532-1541. Fass, D. (2008). The Erv family of sulfhydryl oxidases. Biochim Biophys Acta 1783, 557-566. Grant, C.M. (2001). Role of the glutathione/glutaredoxin and thioredoxin systems in yeast growth and response to stress conditions. Mol Microbiol 39, 533-541. Hansen, R.E., Roth, D., and Winther, J.R. (2009). Quantifying the global cellular thiol-disulfide status. Proc Natl Acad Sci USA 106, 422-427. *Herrmann, J.M., and Dick, T.P. (2012). Redox Biology on the rise. Biol Chem 393, 999-1004. *Herrmann, J.M., and Riemer, J. (2010). The intermembrane space of mitochondria. Antioxid Redox Signal 13, 13411358. Kawano, S., Yamano, K., Naoe, M., Momose, T., Terao, K., Nishikawa, S., Watanabe, N., and Endo, T. (2009). Structural basis of yeast Tim40/Mia40 as an oxidative translocator in the mitochondrial intermembrane space. Proc Natl Acad Sci USA 106, 14403-14407. Kojer, K., Bien, M., Gangel, H., Morgan, B., Dick, T.P., and Riemer, J. (2012). Glutathione redox potential in the mitochondrial intermembrane space is linked to the cytosol and impacts the Mia40 redox state. EMBO J 31, 31693182. Kumar, C., Igbaria, A., D'Autreaux, B., Planson, A.G., Junot, C., Godat, E., Bachhawat, A.K., Delaunay-Moisan, A., and Toledano, M.B. (2011). Glutathione revisited: a vital function in iron metabolism and ancillary role in thiol-redox control. EMBO J 30, 2044-2056. Lee, J., Hofhaus, G., and Lisowsky, T. (2000). Erv1p from Saccharomyces cerevisiae is a FAD-linked sulfhydryl oxidase. FEBS Lett 477, 62-66. *Mesecke, N., Terziyska, N., Kozany, C., Baumann, F., Neupert, W., Hell, K., and Herrmann, J.M. (2005). A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell 121, 1059-1069. Naoe, M., Ohwa, Y., Ishikawa, D., Ohshima, C., Nishikawa, S., Yamamoto, H., and Endo, T. (2004). Identification of Tim40 that mediates protein sorting to the mitochondrial intermembrane space. J Biol Chem 279, 47815-47821. *Riemer, J., Bulleid, N., and Herrmann, J.M. (2009). Disulfide formation in the ER and mitochondria: two solutions to a common process. Science 324, 1284-1287. *Terziyska, N., Lutz, T., Kozany, C., Mokranjac, D., Mesecke, N., Neupert, W., Herrmann, J.M., and Hell, K. (2005). Mia40, a novel factor for protein import into the intermembrane space of mitochondria is able to bind metal ions. FEBS Lett 579, 179-184. Thomazeau, K., Dumas, R., Halgand, F., Forest, E., Douce, R., and Biou, V. (2000). Structure of spinach acetohydroxyacid isomeroreductase complexed with its reaction product dihydroxymethylvalerate, manganese and (phospho)-ADP-ribose. Acta Crystallogr D Biol Crystallogr 56, 389-397. Tienson, H.L., Dabir, D.V., Neal, S.E., Loo, R., Hasson, S.A., Boontheung, P., Kim, S.K., Loo, J.A., and Koehler, C.M. (2009). Reconstitution of the mia40-erv1 oxidative folding pathway for the small tim proteins. Mol Biol Cell 20, 34813490. Toledano, M.B., Delaunay-Moisan, A., Outten, C.E., and Igbaria, A. (2013). Functions and cellular compartmentation of the thioredoxin and glutathione pathways in yeast. Antioxid Redox Signal 18, 1699-1711. Tu, B.P., Kudlicki, A., Rowicka, M., and McKnight, S.L. (2005). Logic of the yeast metabolic cycle: temporal compartmentalization of cellular processes. Science 310, 1152-1158. *Weckbecker, D., Longen, S., Riemer, J., and Herrmann, J.M. (2012). Atp23 biogenesis reveals a chaperone-like folding activity of Mia40 in the IMS of mitochondria. EMBO J. 31, 4348-4358