* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture-08-2013-Bi
Holonomic brain theory wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Single-unit recording wikipedia , lookup
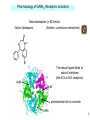
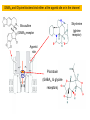
Development of the nervous system wikipedia , lookup
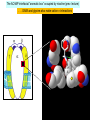
Node of Ranvier wikipedia , lookup
Biological neuron model wikipedia , lookup
Subventricular zone wikipedia , lookup
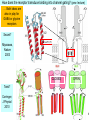
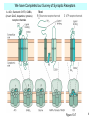
Neuroanatomy wikipedia , lookup
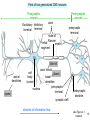
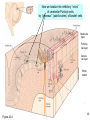
Long-term depression wikipedia , lookup
Electrophysiology wikipedia , lookup
Nervous system network models wikipedia , lookup
Spike-and-wave wikipedia , lookup
NMDA receptor wikipedia , lookup
Apical dendrite wikipedia , lookup
Synaptic gating wikipedia , lookup
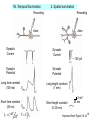
Activity-dependent plasticity wikipedia , lookup
End-plate potential wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Signal transduction wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
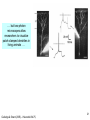
Neuromuscular junction wikipedia , lookup
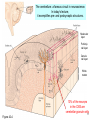
Neurotransmitter wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Synaptogenesis wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
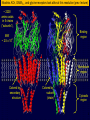
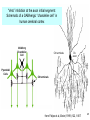
Bi / CNS 150 Lecture 8 Synaptic inhibition; cable properties of neurons; electrical integration in cerebellum Wednesday, October 15, 2013 Henry Lester Chapter 2 (p. 28-35); Chapter 10 (227-232) 1 Nicotinic ACh, GABAA , and glycine receptors look alike at this resolution (prev. lecture) ~ 2200 amino acids in 5 chains (“subunits”), Binding region MW ~ 2.5 x 106 Membrane region Colored by secondary structure Colored by subunit (chain) Cytosolic region 2 The pentameric GABAA and glycine receptors look like ACh receptors; but they are permeable to anions (mostly Cl-, of course) 1. -amino-butyric acid (GABA) is the principal inhibitory transmitter in the brain. 2. Glycine is the dominant inhibitory transmitter in the spinal cord & hindbrain. GABAA receptors are more variable than glycine receptors in subunit composition and therefore in kinetic behavior. . . Cation channels become anion channels with only one amino acid change per subunit, in this approximate location Like a previous lecture A Synapse “pushes” the Membrane Potential Toward the Reversal Potential (Erev) for the synaptic Channels ACh and glutamate receptors flux Na+ and K+, (and in some cases Ca2+), and Erev ~ 0 mV. At GABAA and glycine receptors, Erev is near ECl ~ -70 mV Membrane potential +80 At Erev , the current through open receptors is zero. ENa +60 Positive to Erev, current flows outward +40 Negative to Erev, current flows inward -5 +20 -20 Resting -50 potential -80 EK -100 Like Figure 10-11 4 Pharmacology of GABAA Receptors: activators Benzodiazepines (= BZ below): Valium (diazepam), (Ambien, Lunesta are derivatives) The natural ligand binds at subunit interfeces (like ACh at ACh receptors) phenobarbital site is unceratin 5 The AChBP interfacial “aromatic box” occupied by nicotine (prev. lecture) . . . GABA and glycine also make cation-p interactions aY198 C2 aW149 B aY93 A aY190 C1 non-aW55 D (Muscle Nicotinic numbering) 6 GABAA and Glycine blockers bind either at the agonist site or in the channel Strychnine Bicuculline (glycine receptor) (GABAA receptor Agonist site Picrotoxin (GABAA & glycine receptors) How does the receptor transduce binding into channel gating? (prev. lecture) . . . Both ideas are also in play for GABA or glycine receptors Swivel? Miyazawa, Nature 2003 CLOSED OPEN Twist? Corringer, J Physiol 2010 8 We have Completed our Survey of Synaptic Receptors A. ACh, Serotonin 5-HT3, GABA, (invert. GluCl, dopamine, tyrosine) receptor-channels Most ^ Figure 10-7 9 Parts of two generalized CNS neurons Postsynaptic neuron Presynaptic neuron Excitatory Inhibitory terminal terminal axon node of Ranvier initial myelin segment apical dendrites (apex) little hill axon hillock cell (base) basal body dendrites (soma) presynaptic nucleus terminal presynaptic terminal postsynaptic dendrite synaptic cleft direction of information flow Like Figure 2-1 (rotated) 10 The cerebellum: a famous circuit in neuroscience. In today’s lecture, it exemplifies pre- and postsynaptic structures. Molecular layer Purkinje cell layer Ganule cell layer White matter 10% of the neurons in the CNS are cerebellar granule cells Figure 42-4 11 A plurality of synapses in the CNS (> 1013 ) occur between parallel fiber axons and Purkinje cell dendritic spines Molecular layer 500 nm 12 Types of synapses (Don’t mind the Type I, Type II stuff) Figure 10-3 13 Types of synaptic integration 1. Temporal A. Molecular lifetimes B. Capacitive filtering 2. Spatial 3. Excitatory-inhibitory 14 Previous lecture Synaptic integration 1A. Molecular lifetimes Concentration of high acetylcholine at NMJ (because of 0 acetylcholinesterase, turnover time ~ 100 μs) State 1 closed State 2 k21 all molecules begin here at t= 0 open units: s-1 Number of open channels ms 15 At the nerve-muscle synapse, acetylcholinesterase is present at densities of > 1000 / μm2 near each synapse, high enough to destroy each transmitter molecule as it leaves a receptor What causes the ~ δ-function of glutamate & GABA at CNS synapses? Na+ -coupled transporters for glutamate & GABA are present at densities of > 1000 / μm2 near each synapse, probably high enough to sequester each transmitter molecule as it leaves a receptor (more in a few slides). 16 Synaptic Integration 1B. Capacitive filtering IC C dV Figure 9-6 dT ; V C IC 17 1B. Temporal Summation 2. Spatial summation Recording Recording Axon Axon Synaptic Current Synaptic Current ~ 100 pA Synaptic Potential Synaptic Potential Long time constant (100 ms) Vm Short time constant Vm (20 ms) IC C dV dT ; V C IC Long length constant (1 mm) Short length constant (0.33 mm) 2 mV 25 ms Improved from Figure 10-14 18 1. If dendrites were passive, they would act like leaky cables . . . Excitatory synapses V EPSP measured in soma Gulledge & Stuart (2005) J. Neurobiol 64:75, V EPSP measured in dendrite 19 . . . and passively integrate inputs . . . V V V Δt = 0 Δt = 0 Δt = 5 ms Prolonged rising phase Simultaneous, colocalized EPSPs (two individual trials) Nearly simultaneous, colocalized EPSPs (two individual trials) Simultaneous, Spatially distinct EPSPs Inspect the simulation, and run the movie, at http://www.neuron.yale.edu/neuron/static/about/stylmn.html Gulledge & Stuart (2005) J. Neurobiol 64:75, 20 . . . but two-photon microscopes allow researchers to visualize patch-clamped dendrites in living animals . . . Gulledge & Stuart (2005) J. Neurobiol 64:75, 21 . . . dendrites are not passive. They have Na channels Now break the patch, to fill the cell with dye: * = axon hillock Averaged traces 25 μm Magee & Johnston, J Physiol (1995) immunocytochemistry Whitaker, Brain Res, 2001 22 brain slice . . . voltage-gated Na+ and Ca2+ channels in dendrites lead to partial “backpropagation” of action potentials, implying that parts of cells can process signals semi-independently. Stay tuned! Gulledge & Stuart (2005) J. Neurobiol 64:75, 23 3. Excitatory-inhibitory integration: The “veto principle” of inhibitory transmission Inhibitory synapses work best when they are “near“ the excitatory event they will inhibit. “Near” means < one cable length. A. Inhibitory synapses on dendrites do a good job of inhibiting EPSPs on nearby spines B. Inhibitory synapses on cell bodies and initial segments do a good job of inhibiting spikes 24 “Veto” inhibition at the axon initial segment: Schematic of a GABAergic “chandelier cell” in human cerebral cortex Inhibitory Chandelier Cell Ch terminals Ch. axon Pyramidal Cells Ch terminals from Felipe et al, Brain (1999) 122, 1807 25 Now we localize the inhibitory “vetos” of cerebellar Purkinje cells by “pinceaux” (paintbrushes) of basket cells Molecular layer Purkinje cell layer Ganule cell layer White matter Figure 42-4 26 How to localize and quantify inhibitory synapses NH2 A fusion protein: GABA transporter (GAT1)-GFP 27 cerebellum 28 <Immunocytochemistry For GABA transporter Molecular layer (basket cells stain) Purkinje cell layer “pinceux” (paintbrushes) stain heavily Granule cell layer 29 <Immunocytochemistry For GABA transporter mGAT1 GFP knock-in fluorescence > Molecular layer (basket cells stain) Purkinje cell layer “pinceaux” stain heavily, showing soma-hillock “veto” Granule cell layer 30 GAT1-GFP expression in cerebellum: basket cell terminals in molecular layer, Showing dendritic “veto” GABA transporter density is ~1000/(μm2) 50 mm 31 End of Lecture 8 32