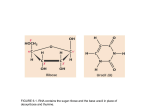
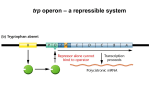
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Landick R, Yanofsky C. 1987. Transcription
Paracrine signalling wikipedia , lookup
RNA interference wikipedia , lookup
Real-time polymerase chain reaction wikipedia , lookup
Biochemistry wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Non-coding DNA wikipedia , lookup
Proteolysis wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Peptide synthesis wikipedia , lookup
Polyadenylation wikipedia , lookup
RNA silencing wikipedia , lookup
Messenger RNA wikipedia , lookup
Expression vector wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Biosynthesis wikipedia , lookup
Gene regulatory network wikipedia , lookup
Transcription factor wikipedia , lookup
Point mutation wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Epitranscriptome wikipedia , lookup
Genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Gene expression wikipedia , lookup
Eukaryotic transcription wikipedia , lookup
RNA polymerase II holoenzyme wikipedia , lookup
77. Transcription Attenuation ROBERT LAN DICK AND CHARLES YANOFSKY Department of Biological Sciences, Stanford University, Stanford, California 94305 BRIEF HISTORICAL REVIEW ............................................................................... 1276 ORGANIZATION OF THE LEADER REGIONS OF AMINO ACID BIOSYNTHETIC OPERONS REGULATED BY TRANSCRIPTION ATTENUATION ...................................................................................................... 1277 MODEL OF THE MECHANISM OF TRANSCRIPTION ATTENUATION CONTROL OF THE E. COLI tIp OPERON ........................................................ 1278 EVIDENCE FOR INDIVIDUAL FEATURES OF ATTENUATION IN THE tIp OPERON ........................................................................................................ 1278 Translation of the Leader Peptide Coding Region .............................................. 1278 Functions of the Terminator and Attenuator ...................................................... 1280 Roles of the Transcript Pause and Antiterminator Secondary Structures ...... 1281 RNA Polymerase Recognition of Pause and Termination Signals .................... 1281 FINE FEATURES OF ATTENUATION IN THE tIp OPERON ........................... 1281 Synchronization of Transcription and Translation ............................................ 1281 Transcription Readthrough in the Presence of Excess Tryptophan ................. 1282 Shutdown of Leader Peptide Synthesis ................................................................ 1282 Starvation for Amino Acids Other than Trp, and Significance of the Two Trp Codons in the Leader Transcript ............................................................... 1283 TRANSCRIPTION ATTENUATION IN OTHER AMINO ACID BIOSYNTHETIC OPERONS ............................................................................. 1283 The his Operon ........................................................................................................ 1283 Regulation of the his operon .............................................................................. 1283 Sequence of the his leader region ...................................................................... 1283 Detection of the terminated his leader transcript ........................................... 1283 Demonstration that synthesis of the his leader peptide is required for attenuation ........................................................................................................ 1283 Mutations altering the leader peptide ............................................................... 1284 Mutations affecting his leader transcript secondary structures .................... 1285 Mutations affecting tRNA Hls synthesis ............................................................. 1285 The leu Operon ........................................................................................................ 1286 The thr Operon ........................................................................................................ 1286 The ilvGMEDA Operon .......................................................................................... 1290 The ilvBN Operon ................................................................................................... 1290 The pheA Operon .................................................................................................... 1291 LEADER PEPTIDE SEQUENCES AND FUNCTIONS ......................................... 1291 TRANSCRIPTION ATTENUATION IN NON-AMINO ACID BIOSYNTHETIC OPERONS ............................................................................................................. 1292 The pheST Operon .................................................................................................. 1292 Pyrimidine Biosynthetic Operons ......................................................................... 1292 The ampC Operon ................................................................................................... 1294 rRNA Operons ......................................................................................................... 1295 Ribosomal Protein Operons ................................................................................... 1295 The tna and liv Operons ......................................................................................... 1296 CONCLUSIONS .......................................................................................................... 1297 ACKNOWLEDGMENTS ............................................................................................ 1297 LITERATURE CITED ................................................................................................ 1297 BRIEF HISTORICAL REVIEW cantly increased the rate of synthesis of histidine biosynthetic enzymes (148). Studies on the mechanism of action of this analog suggested that it was an inhibitor of tRNAHis charging. At about the same time it was observed that temperature-sensitive valyl-tRNA synthetase mutants had elevated levels of valine biosynthetic enzymes (40), similarly implicating tRNA One of the earliest observations suggesting that tRNA function was involved in regulating expression of amino acid biosynthetic operons was the finding that addition of the histidine analog a-methylhistidine to cultures of Salmonella typhimurium signifi1276 TRANSCRIPTION ATTENUATION 77 charging in operon-specific regulation. Following these early studies, other investigations with the his operon established that mutations that altered tRNAHis structure or function affected his operon regulation (21). Although the possibility was considered that a repressor protein existed that recognized either charged or uncharged tRNA His , mutational studies aimed at detecting such a regulatory protein were unsuccessful (21, 108). Many subsequent analyses focused on a particular re~ulatory mutant that produced undermodified tRNA is; observations with this strain, like those with a-methylhistidine, implicated translation of His co dons in the regulation of his operon expression (6, 21, 169). Further studies with this and other mutants culminated in the hypothesis that translation of the early portion of the transcript of the his operon was involved in his operon regulation (R. G. Martin, B. N. Ames, and P. E. Hartman, Int. Congo Biochem. Abstr. 2:261, 1967; R. G. Martin and B. Ames, personal communication). During this period, investigations with the trp operon of Escherichia coli were concerned principally with analyses of tryptophan-mediated repression of trp operon expression. At the time it was thought that repression was the sole transcription regulatory mechanism controlling this operon's expression (28, 81); however, a number of findings were inconsistent with this view. For example, the rate of trp mRNA synthesis markedly increased when a mutant strain lacking a functional trp repressor was subjected to severe tryptophan starvation (9). This would not be expected if repression were the sole transcription control mechanism specifically responsive to tryptophan. It also was noted that expression of the operon was elevated in strains with a defective trY,frtophanyl-tRNA synthetase (119), implicating tRNA rp or its cognate synthetase in trp operon regulation. An additional unexplained finding was that transcription in progress over the initial segment of the trp operon appeared to terminate abruptly when tryptophan was added to tryptophan-starved, repressor-deficient cultures (74, 77). These and related findings with the trp and his operons--observations that were not readily explicable by repression-assumed greater significance when genetic evidence established the existence of regulatory sites within the initially transcribed regions of these operons that limited their expression. With both operons it was shown that expression was significantly increased by deletions that removed the initial segment of the operon and left the respective promoters intact (80, 90). mRNA measurements proved that this increase was transcriptional and suggested that a transcription termination site was located in the distal segment of the leader regions of both operons; the deletions that increased operon expression were presumed to do so by removing this termination site (16, 17,80,90). A unifying hypothesis was rapidly evolving, namely, that specific tRNA charging, mRNA translation, or both provided the basis for the decision whether or not to terminate transcription in the leader regions of these operons. This view was solidified when the leader regions of the trp, his, and other amino acid biosynthetic operons were sequenced and each was shown to contain a short peptide coding region rich in 1277 co dons for the regulating amino acid(s). Two Trp co dons were found in the coding region for the trp leader peptide (106), seven Phe codons were present in the pheA leader peptide coding region (186), and seven contiguous His codons were located in the coding region for the his leader peptide (10, 38). In addition, in the ilvGMEDA operon, which is regulated by isoleucine, valine, and leucine, there were several codons for each of these amino acids in the leader peptide coding region (103, 121). The existence of coding regions rich in codons for the regulating amino acid(s) constituted indisputable evidence implicating specific tRNAs and translation in transcription regulation. Additional support was provided by the key observations that ribosomes could bind to the presumed translation initiation site for the trp leader peptide (134) and that transcription terminated in the trp leader region in vivo and in vitro (15, 16, 105). The case for translational control of transcription termination in the leader regions of amino acid biosynthetic operons rapidly gained overwhelming experimental support. In the ensuing years a substantial body of information has been gathered establishing that transcription attenuation is commonly used to regulate amino acid biosynthetic operons (13, 132, 175). In addition, operons concerned with a variety of metabolic functions have been found to be regulated by transcription attenuation. Mechanistic studies with amino acid biosynthetic operons have elucidated the major events involved in transcription attenuation, providing us with a thorough understanding of this regulatory phenomenon. ORGANIZATION OF THE LEADER REGIONS OF AMINO ACID BIOSYNTHETIC OPERONS REGULATED BY TRANSCRIPTIO:\[ ATTENUATION The structural features of the leader regions of amino acid biosynthetic operons regulated by attenuation are surprisingly similar. These features include sites of transcription pausing and Rho-independent transcription termination, a coding region for a short leader peptide containing codons for the regulating amino acid(s), and segments that specify alternative RNA secondary structures (Fig. 1). Transcription pausing occurs early in the leader region; pausing is due to the formation of an RNA structure, namely, the transcription pause signal. Distally located in the leader region is the attenuator, the DNA site of regulated transcription termination. The attenuator encodes an RNA secondary structure, i.e., the transcription termination signal or terminator. Overlapping ATTENUATION CONTROL REGION PROMOTER transcri pt ATTENUATOR pause structure 1 2 FIRST STRUCTURAL GENE tenn; nator 3 4 t ant; term; nator pept; de codi n9 r-eg" on FIG. I. Structure and relevant features of the leader regions and leader transcripts of amino acid biosynthetic operons regulated by transcription attenuation. 1278 LAN DICK AND YANOFSKY the terminator and the pause structure is an alternative RNA secondary structure termed the antiterminator. Formation of this structure during transcription of the leader region precludes formation of the terminator, thereby permitting transcription readthrough. The peptide coding region usually overlaps the promoter-proximal RNA segment that is part of the RNA pause signal. Translation of the peptide coding region versus ribosome stalling within it selects between terminator and antiterminator formation, causing either transcription termination or transcription readthrough. In this article we shall begin by describing attenuation in the trp operon in detail, to illustrate the individual features of transcription attenuation as they are currently understood. We shall then consider other examples of transcription attenuation in amino acid biosynthetic operons, highlighting features that are unique to each. Finally, we shall discuss examples of transcription attenuation in E. coli and S. typhimurium that involve mechanisms substantially different from those operating in amino acid biosynthetic operons. MODEL OF THE MECHANISM OF TRANSCRIPTION ATTENUATION CONTROL OF THE E. COLI trp OPERON Attenuation in the trp operon is a dynamic process involving both transcription and translation. In E. coli cultures growing at 3rC, transcription initiation at the trp promoter can occur as often as once every 6 s (9,16). At this temperature, each transcribing polymerase molecule theoretically can reach the attenuator in 2 to 3 s. It follows, therefore, that for attenuation to be an effective regulatory mechanism, the individual events crucial to attenuation must be rapid and must be precisely timed. In this section we shall describe the sequence of events, as we believe they occur, in attenuation control of trp operon expression. A schematic representation of these events is depicted in Fig. 2. In the trp operons of E. coli and S. typhimurium, repression and attenuation function as independent transcription control mechanisms (see chapter 90). Tryptophan activates the trp aporepressor; the resulting repressor can bind at the trp operator and inhibit transcription initiation. Under conditions of maximum repression, the rate of transcription initiation at the trp promoter is about 1/80 the rate observed in the absence of tryptophan (79, 179). All polymerase molecules that initiate transcription on the trp operon are subject to a transcription termination decision at the attenuator. This decision is based on the cellular level of charged tRNA Trp. Under starvation conditions, in which all tRNATrp essentially is uncharged, the rate of read through transcription at the attenuator increases about eightfold over the rate observed when tRNATrp is fully charged. We do not believe that the rate of transcription initiation at the trp promoter influences attenuation control, or vice versa, but these possibilities have not been excluded. The first event crucial to attenuation is formation of RNA secondary structure 1:2 (Fig. 2). This structure functions as a transcription pause signal and presumably forms rapidly and spontaneously as the tran- scribing polymerase molecule moves over the initial segment of the leader region. While the polymerase pauses in the leader region, the opportunity is provided for loading of a ribosome at the leader peptide ribosome-binding site of the leader transcript. After a ribosome loads and begins translation, it approaches the paused polymerase. As it nears the paused polymerase it could disrupt the RNA pause structure, releasing the polymerase and restoring transcription. Polymerase and ribosome then move in unison over the leader region and leader transcript, respectively. At this stage there are two options (176). If there is a deficiency of charged tRNA Trp, the translating ri bosome would stall at one of the Trp codons in the peptide coding region. If this occurred, then RNA segment 1 would be masked by the stalled ribosome. This would allow RNA segment 2 to pair with RNA segment 3 as soon as that RNA segment was synthesized. (Paired RNA segments 2 and 3 constitute the anti terminator.) As transcription continues and RNA segment 4 is formed, the antiterminator will persist, preventing formation of the terminator. Since the terminator does not form, the transcribing polymerase molecule will read through the attenuator and transcribe the remainder of the operon. Alternatively, if there is an adequate level of charged tRNATrp, the ribosome translating the leader peptide coding region would continue beyond the Trp codons to the end of the coding region and then either remain at the translation stop codon (as shown in Fig. 2) or dissociate from the leader transcript. If the ribosome remains at the stop codon while RNA segment 4 is synthesized, the ribosome would block antiterminator (2:3) formation and promote terminator (3:4) formation, leading to transcription termination. For reasons that will become evident, it is thought that if the ribosome releases quickly at the stop codon the transcript will usually refold to form structure 1 :2. When 1:2 forms, then structure 3:4, the terminator, subsequently will form, and transcription will terminate at the attenuator. However, after ribosome release at the stop codon, structure 2:3 occasionally may form if segment 3 has been synthesized. If 2:3 does form, it would prevent formation of the terminator, as it does during tryptophan starvation. Attenuation in the trp operon therefore permits the cell to exploit the position of the ribosome engaged in synthesis of the trp leader peptide as the basis for the decision whether or not to terminate transcription at the attenuator. Whenever the terminator is formed during transcription of the leader region, it serves as a termination signal, and RNA polymerase terminates transcription at the attenuator. Whenever the anti terminator forms, transcription proceeds into the operon. EVIDENCE FOR INDIVIDUAL FEATURES OF ATTENUATION IN THE trp OPERON Translation of the Leader Peptide Coding Region Attenuation allows the bacterium to regulate transcription and ultimately translation of the trp operon structural genes as a function of charged tRNA Trp availability. Conditions and events that reduce tRNATrp charging increase operon expression by reducing termination at the attenuator. The extent of tRNATrp charging TRANSCRIPTION ATTENUATION 77 1279 INITIAL STAGES OF TRANSCRIPTION ~ - POLYMERASE PAUSES + ppp/AUG 1:2 RIBOSOME BINDS TO TRANSCRIPT I ~, =r:f="" - MOVING RIBOSOME RELEASES THE PAUSED POLYMERASE AAA PPP--AUG / -- TRYPTOPHAN-STARVED CULTURE RIBOSOME STALLS AT A TRP CODON, ANTITERMINATOR FORMS ~ GROWTH WITH EXCESS TRYPTOPHAN RIBOSOME MOVES TO THE STOP CODON l 1 TERMINATOR CANNOT FORM, TRANSCRIPTION CONTINUES TERMINATOR FORMS READ- THROUGH TERMINATION FIG. 2. Model depicting the events involved in regulation of the trp operon by transcription attenuation. The ribosome remains attached to the nascent RNA after it reaches the leader peptide stop codon, although this feature of the attenuator model remains unclear (see text). is dependent on the availability of tryptophan, the rate of tRNATrp charging, and the overall rate of protein synthesis. Conditions that deplete the pool of charged tRNA Trp, such as tryptophan starvation or rapid protein synthesis, increase readthrough at the attenuator (17, 120). Similarly, all changes that reduce the efficiency of Trp codon translation increase operon expression. These include alterations in the structural gene for tRNATrp or tryptophanyl-tRNA synthetase (119,180) or alterations in the modifying enzyme that adds the isopentenyl group to the A residue adjacent to the anticodon of tRNATrp (41, 42, 170, 180). The discovery of a ribosome-binding site in vitro (134) and its correspondence to a potential translation initiation site in the leader segment of the trp transcript (104) provided the initial basis for the suggestion that the leader peptide coding region was translated. The first proof of this hypothesis was provided by gene fusion analyses. The trp leader peptide coding region was fused in phase to the lac! coding region (155) and to the distal segment of trpE (117). In both instances a fusion polypeptide was synthesized, and amino acid sequence analyses verified that translation had begun at the leader peptide start codon. These observations supported the idea that the signal for attenuation control must include the ability to translate the tandem Trp codons in the leader transcript. The importance of translation of the leader peptide coding region to regulation by attenuation is also shown by the effects of alterations in the leader region that reduce or eliminate its translation. Replacement of the AUG start codon for toe leader peptide by AUA (trpL29; Fig. 3A) essentially eliminates the relief of termination at the attenuator that normally accompanies tryptophan starvation (32, 187). Similarly, deletions that replace the Shine-Dalgarno sequence preceding the leader peptide start codon, or replace this sequence and the start codon, prevent the typical tryptophan starvation response (163, 164). Therefore, both the capacity to translate the leader peptide coding region and, more specifically, the ability or inability to translate the tandem Trp codons in this message segment are essential to regulation by attenuation. Attempts to detect synthesis of the trp leader peptide in vivo were unsuccessful until recently (37). However, the elusive peptide was detected in vitro in analyses using a coupled transcription-translation system (33). These studies also provided explanations for the lack of success in early attempts to find the peptide. Turnover measurements in vitro demonstrated that the trp leader peptide is extremely labile. It also was shown that synthesis of the leader peptide was depressed when the template contained the segment of the leader region just beyond the peptide coding region. The corresponding segment of the transcript can fold back and form a base-paired structure that blocks the translation start site (33). Experiments exploiting these facts have demonstrated synthesis of the trp leader peptide in vivo (37). 1280 LANDICK AND YANOFSKY 1:2 A A A UI St op IA C G ~ A trpL75,76,77 trpL67,6B U ~C=G~ Ser C=G AAGUUCACG U U A A A A Arg G trpL29 A ~ Trp t Trp A trpLBO C G 3:4 A U 60 C G G U A U-A . / c=G---8o A-U C=G Thr A 20 A G U A U C A U A G U 120 C=G G U G=C G=C trpLIIB A.- C=G-A trpL130 g:g=:; ~:~ C Met~YSAlaIlePheValLeULYSGlyG=C GACAAUGAAAGCAAUUUUCGUACUGAAAGGUU-A 40 trpL131 trpL132 G=C AUCAGAUACCCA-UUUUUUU U-A 140 P""""G=C C=G 100 G A U A A 2:3 A B U A G AAGUUC A C=G 100 A C trpL93 A~G=C U-A G U A A A A A A U C A A C=G U-A U A-U U-A G=C G G G U 20 A A C C U AI C GA AC U trpL75,76,77 A " C=G--A trpLl15 C A ~ G=C G G=C A MetLysAlaIlePheValLeuLysGlyTrpTrpArgThrSerStop G=C CAAUGAAAGCAAUUUUCGUACUGAAAGGUUGGUGGCGCACUUCCUGAAAC=GCCUAAUGAGCGGGCUUUUUUU 40 60 120 140 FIG. 3. Alternative secondary structures of the E. coli trp leader transcript. The 1 :2,2:3, and 3:4 secondary structures were predicted by a computer algorithm (185) and are consistent with the observed pattern of RNase TJ partial digestion (129). The positions of key mutations are indicated. (A) Termination configuration. Site of transcription pausing (49) is indicated by the boldface arrow. (B) Antitermination configuration. Functions of the Terminator and Attenuator The distal portion of the trp leader region (the attenuator) specifies a transcript segment that can fold to form the key structure, the terminator (3:4; Fig. 3A). The base-paired segment of this RNA structure is followed by a run of U residues that constitute the 3' end of the terminated transcript. The importance of this segment of the leader region to transcription termination is demonstrated by mutational analyses which have shown that alteration of specific base pairs in this region (trpL118, trpLJ 3D, trpLJ 31, trpLJ 32; Fig. 3A) or deletion of any of its segments eliminates transcription termination at the attenuator in vivo and in vitro (159, 162, 164, 188). These mutational changes undoubtedly exert their effect by altering the structure of the corresponding transcript segment. It is thought that the terminator is the transcription stop signal recognized by the transcribing polymerase molecule (45, 133, 144, 146). This conclusion is based on several experiments that distinguish between template and transcript as the termination signal. Analyses involving incorporation of base analogs (that decrease or increase base-pair stability) into the transcript or DNA template suggested that the termination signal resided within the RNA itself rather than within the corresponding DNA segment (45, 47,106). The final compelling evidence was obtained with DNA heteroduplexes prepared from the separated strands of the leader regions of wild type 77 and a mutant with an attenuator alteration that reduces termination (146). One of the heteroduplex templates specified a wild-type transcript while the other specified a mutant transcript. Transcription studies with these templates showed that only when the mutant transcript was produced was termination relieved (146). When restriction fragments containing the trp leader region were transcribed in vitro with purified RNA polymerase, two major transcripts were detected (105, 106, 129). Both were initiated at the trp promoter. One, the so-called leader transcript, terminated at about base pair 140 in the leader region, the position of in vivo termination, while the other, the runoff transcript, extended to the end of the restriction fragment (15,106). The predominant product was the leader transcript; at 32°C and above, approximately 95% of transcripts initiated at the trp promoter terminated in the leader region (45, 50, 174), whereas at lower temperatures, the percentage readthrough increased to about 25% (45, 174). Experiments performed in vitro showed that the transcription termination complex dissociated spontaneously at the trp attenuator, releasing transcript, template, and polymerase (14). Accessory complexbinding factors therefore do not appear to be essential for this dissociation. However, it seems likely that in vivo such factors interact with the complex or one of its components and facilitate transcript release. One candidate for such a protein is the NusA protein (59). However, this protein did not influence transcription termination at the trp attenuator when added to transcription reaction mixtures containing trp operon templates (44, 50, 174). A second transcription accessory factor, the Tau protein, appears to influence termination complex dissociation with other systems (23). Roles of the Transcript Pause and Antitermim.tor Secondary Structures The segment of the leader transcript just beyond the translation start codon can form the stable hairpin designated 1:2 (Fig. 3A). When this transcript segment is synthesized by the transcribing polymerase molecule, polymerase pauses on the template and forms a paused transcription complex (46, 91,174). This complex never terminates transcription (49). The longevity of the paused complex is influenced markedly by the concentration of the next nucleotide to be added, as the paused polymerase resumes transcription, and by the presence of the NusA protein (44, 46, 49-52, 101,174). Paused complex release is promoted by high concentrations of this nucleotide and inhibited by the presence of the NusA protein. It is thought that RNA hairpin 1:2 serves as the pause signal. This conclusion is based on analyses with mutant templates which specify transcripts with pause structures with reduced stability (trpL67,68, trpL75,76,77, trpL80; Fig. 3A), and on studies employing nucleotide analogs as transcription substrates, and is supported by the demonstration in vitro that the paused complex is released by the addition of an oligonucleotide complementary to the trailing strand of the RNA hairpin (50,52, 101, 173). RNA segments 2 and 3, which form parts of the pause and terminator hairpins, also may form the TRANSCRIPTION ATTENUATION 1281 antiterminator. This structure is not observed in vitro in the intact leader transcript, but it is readily detected in transcripts of deletion mutants in which the regions corresponding to the competing segments (1 and 4) of the pause and terminator structures have been removed (98). Studies in vivo and in vitro with leader point mutants (trpL75,76,77, trpL93, trpL115; Fig. 3B) and deletions in which the stability of the anti terminator is reduced establish that this structure causes termination relief. Deletions which remove only RNA segment 1 and thereby prevent 1:2 formation show little or no termination in vivo or in vitro (162-164). However, if the deletion removes part of segment 2 as well, thus preventing the antiterminator from forming, then efficient termination at the attenuator is observed (162-164). When RNA segments 1,2, and 3 are deleted, there is no termination at the attenuator either in vivo or in vitro (162-164). These findings establish that termination and termination relief are consequences of the formation of different structures in the leader transcript and reinforce the conclusion that the role of the translating ribosome is to determine which of the two structures forms. RNA Polymerase Recognition of Pause and Termination Signals Mutants have been isolated that produce altered RNA polymerases that behave aberrantly in the recognition of transcription pause and termination signals (51, 178). The mutants studied to date were isolated as rifampin resistant, and all are altered in rpoB, the structural gene for the J) subunit of RNA polymerase. The mutants were recognized by their increased or decreased ability to terminate transcription at the trp attenuator in vivo. When RNA polymerase was purified from these mutants and studied in vitro, the altered termination behavior seen in vivo was reproduced (51). These mutant polymerases also behave aberrantly in transcription pause analyses (51). Mutant polymerases that terminated transcription at the attenuator more efficiently formed more stable paused complexes. Conversely, mutant polymerases that terminated less efficiently produced more short-lived paused complexes. These observations indicate that the polymerase alterations studied comparably affect the response to both pause and termination structures. The participation of the other polymerase subunits in the response to pause and termination structures has not been examined. FINE FEATURES OF ATTENUATION IN THE trp OPERON Synchronization of Transcription and Translation The model of attenuation in amino acid biosynthetic operons suggests that each leader transcript must load a ribosome and that this ribosome must synthesize all or part of the leader peptide while the transcribing RNA polymerase molecule is within the leader segment of the operon, i.e., the segment that specifies alternative RNA secondary structures. Comparative mRNA measurements with wild-type strains and with deletion mutants that lack the attenuator have shown that during severe tryptophan starvation 1282 LAN DICK AND YANOFSKY there is little or no termination at the attenuator (17, 164). Thus, if the attenuation model is correct, bacteria starved of tryptophan must have a ribosome stalled over one of the Trp co dons of every leader transcript. This could be accomplished if ribosome loading at the leader peptide ribosome-binding site were extremely rapid, or if transcription were delayed at the pause site in the leader region until the paused polymerase was released by the translating ribosome. The available evidence favors the latter possibility (100). As mentioned earlier, the first RNA hairpin structure to form in the leader transcript functions as a transcription pause signal (46, 49, 101, 174). Experiments performed using an in vitro coupled transcriptiontranslation system indicate that the paused transcription complex is released by the moving ribosome as the leader peptide coding region is being translated (loa). Pause release occurs much more slowly when translation of the leader peptide coding region is prevented. Release is observed under tryptophan starvation and nonstarvation conditions (100). This is expected, since the Trp codons are within the segment of the transcript that forms the RNA pause structure. Thus, transcription pausing, and pause release by the translating ribosome, may be the events that synchronize transcription of the leader region with translation of the coding region for the leader peptide. Transcription Readthrough in the Presence of Excess Tryptophan When E. coli cultures are grown in the presence of excess tryptophan, 1 of every 5 to 10 polymerase molecules that transcribe the leader region does not terminate at the attenuator (16, 179). Similarly, during growth with excess tryptophan, repression permits a transcription initiation frequency 1/S0 the rate obtained in the absence of repression (179). Conceivably the low level of trp operon transcription maintained in the presence of excess tryptophan equips the cell with sufficient levels of the trp enzymes so that small amounts of tryptophan could be synthesized if a culture growing in tryptophan excess were shifted abruptly to a tryptophan-free environment. How is the low level of transcription read through at the trp attenuator maintained in cultures growing with excess tryptophan? The available evidence suggests that in tryptophan excess, read through is influenced both by translation of the leader peptide coding region and by the relative stabilities of the RNA pause, anti terminator, and terminator structures (97,101,163). Base-pair changes and deletions in the leader region that reduce or eliminate translation of the leader peptide coding region result in fivefold greater termination at the attenuator in vivo in excess tryptophan than is observed in strains with the wild-type operon (163, 187). This phenomenon has been termed superattenuation (163). Since in some of these mutants the transcript secondary structures are unaltered, increased termination must be a direct consequence of the translational defect. These findings suggest that in the absence of translation the RNA terminator must form more frequently. We explain this by assuming that, in the absence of translation, the pause structure forms normally and causes pausing, but when the paused polymerase resumes transcription, reformation of the 1:2 pause structure prevents formation of the anti terminator. If the anti terminator does not form, the terminator willand cause termination. The fivefold higher level of readthrough observed in wild type in vivo when there is translation probably is due to the more frequent formation of the antiterminator. There are two ways at least that this could be accomplished (97, 101). After the moving ribosome disrupts the pause structure, it could occasionally remain near the Trp co dons on segment 1 long enough to favor formation of the anti terminator and allow RNA polymerase to transcribe past the attenuator. Alternatively, the translating ribosome might move rapidly to the leader peptide stop codon. If so, the basal level of read through would be determined by how fast the ribosome releases at the stop codon: a key question to which there is no answer at present. If the ribosome releases before segment 3 is synthesized, 1:2 reformation would lead to termination. If segment 3 is synthesized before release, 1:2 and 2:3 would compete for formation. Thus, the probability of read through might be determined by the location of the transcribing polymerase when the ribosome dissociated and by the relative probabilities of formation of the competing RNA structures. Analyses with mutants that form altered pause or antiterminator structures have shown that the stabilities of these structures do influence the extent of readthrough transcription in vivo in the presence of excess tryptophan (97, 101). Since the relative stabilities of the trp pause and anti terminator structures vary greatly in different enterobacteria, we suspect that there may be a wide range of basal read through levels in these species (177). This could reflect the different environmental experiences each species has had to adjust to during its evolution. Shutdown of Leader Peptide Synthesis The terminated trp leader transcripts of the several enterobacterial species that have been studied have a segment near their 3' end that theoretically can base pair with the ribosome-binding site used in the synthesis of the leader peptide and thus block its utilization in translation initiation (177). Structural analyses with isolated leader RNAs of S. typhimurium and Serratia marcescens have shown that the leader ribosome-binding site can in fact base pair with this distal segment of the transcript (98; K. Brown and C. Yanofsky, unpublished data). Experiments designed to test whether this pairing blocks synthesis of the leader peptide have been performed using a highly purified DNA-dependent dipeptide-synthesizing system and S. typhimurium trp leader templates that do or do not contain the segment complementary to the leader ribosome-binding site. It was observed that synthesis of the leader initiation dipeptide, fMet-Ala, was reduced about lO-fold when the template contained the downstream complementary region (33). We assume from these findings that leader peptide synthesis is shut down in vivo soon after the decision is made whether or not to terminate transcription at the attenuator. 77 Starvation for Amino Acids Other than Trp, and Significance of the Two Trp Codons in the Leader Transcript There are two Trp co dons in the coding region for the 14-residue trp leader peptide. Does ribosome stalling at other co dons in this coding region also relieve attenuation? This question has been addressed in amino acid starvation experiments performed with E. coli cultures containing the E. coli or S. marcescens trp leader region (164, 187). It was observed that with few exceptions, starvation for amino acids corresponding to other co dons in the trp leader transcript did not relieve attenuation. The interesting exceptions involved starvation for arginine, the amino acid specified by the codon immediately following the Trp codons, and starvation for histidine in studies with the S. marcescens leader region. In the trp leader peptide coding region of the latter organism there is a single His codon two co dons before the Trp codons. These findings indicate that the position of the stalled ribosome on the transcript is crucial to attenuation relief and that the critical segment of the trp transcript extends beyond the two Trp codons. If one assumes that a stalled ribosome masks about 13 nucleotides on either side of the codon which is being read (175, 177), then stalling at the Arg, His, or Trp codons of the leader transcript would be expected to promote formation of the anti terminator. Therefore the results of these experiments illustrate the importance of codon location, as well as the number of the various leader regulatory codons, to regulation by attenuation. TRANSCRIPTION ATTENUATION IN OTHER AMINO ACID BIOSYNTHETIC OPERONS The his Operon Regulation of the his operon. The E. coli and S. typhimurium his operons each are composed of nine genes that encode the enzymes required for the synthesis of histidine from ATP and phosphoribosylpyrophosphate (7,18,21; see also chapter 25). In the 1960s it was recognized that the availability of histidine regulated the synthesis of the histidine biosynthetic enzymes in these organisms (145; see Brief Historical Review, above). By the early 1970s it was recognized that translation was involved in his operon regulation (Martin et aI., Int. Congo Biochem. Abstr. 2:261,1967; Martin and Ames, personal communication), and histidine-specific transcription attenuation was postulated (90). In the late 1970s and early 1980s, DNA sequence analyses and mutational studies with the E. coli and s. typhimurium his operon leader regions led to a detailed, well-supported model for the regulation of the his operon by attenuation. Because the large body of mutational data on regulation of the his operon provided important underpinnings for the current general model of attenuation in amino acid biosynthetic operons, we shall describe studies with this operon in greater detail than other examples of attenuation. Sequence of the his leader region. The sequences of the E. coli (38) and S. typhimurium (10) his operon leader regions were reported in 1978. The sequence TRANSCRIPTION ATTENUATION 1283 data immediately suggested a model for his operon attenuation that was compatible with previous regulatory findings. The following significant features were identified: (i) the his leader regions of both species encode an identical 16-residue leader peptide that contains 7 contiguous histidine residues, at positions 8 through 14; (ii) segments of the leader transcript theoretically can fold into two alternative secondary structures: one has a potential Rho-independent terminator at its 3' end, and the other structure could function as an anti terminator, precluding formation of the terminator; and (iii) the location of the seven His co dons in the leader peptide coding region would allow a ribosome stalled at these codons to promote formation of the anti terminator and hence prevent formation of the terminator. If the model based on these features is correct, mutational changes in the leader peptide coding region or the base-paired segments that form alternative RNA secondary structures should have profound effects on regulation of the his operon. The alternative secondary structures proposed to regulate attenuation in the his leader region (Fig. 4A and B) have features that differ only slightly from those formed by the trp leader transcript. The his leader transcript can form three hairpin structures in the termination configuration, 1:2, 3:4, and 5:6 (Fig. 4A), whereas in the alternative, anti termination configuration (Fig. 4B), segment 2 (available when a ribosome stalls on segment 1) can pair with segment 3, thereby allowing structure 4:5 to form and block formation of the terminator (5:6). Detection of the terminated his leader transcript. Both the E. coli and S. typhimurium his leader transcripts have been identified as products of in vitro transcription (53, 60). These transcripts originate at homologous positions, and the promoter sequences for the operons are nearly identical. Termination of the his leader transcript in vitro is extremely efficient; significant readthrough at the S. typhimurium attenuator occurs only when ITP replaces GTP in the reaction mixture, presumably reducing the stability of the 5:6 hairpin (53). A transcription pause after synthesis of the 1:2 hairpin structure has not been reported, but seems likely. Demonstration that synthesis of the his leader peptide is required for attenuation. Constitutive mutations that affect the rate of initiation of leader RNA synthesis have never been obtained. This is in accordance with the attenuation model, since His regulation only affects termination at the attenuator. To isolate leader peptide mutants incapable of responding to His starvation (termed hisO P mutations), 10hnston and Roth (87) devised a selection for decreased his operon expression. This selection was based on the adenine auxotrophy induced by ATP consumption in a strain with elevated his enzyme levels (hisT -) and a feedback-resistant hisG protein. Mutations causing adenine prototrophy and decreased his operon expression result in His auxotrophy (strong phenotype) or permit strains to remain His+ but become sensitive to aminotriazole (AT', weak phenotype). This selection was used to obtain a large number of mutations that limited expression of the his operon (88, 89). 10hnston and Roth (89) described five different mutations that reduce his operon expression by affecting translation initiation of the leader peptide coding LANDICK AND YANOFSKY 1284 A AUCAAAUGA 1:2 A U G U Stop A A A G ASp U A A-U A Pro U A A His C C~G his09675 UGACACGC His ~+.~ U-A A-U HiS::: MetrhrAr~alGlnpheLYSHiSHiSHiSHiSA-U A-U A-U U C U A C~G C~G G~C G~C G C~G C A A-U C~G C~G G~C A-U U U G~C 3:4 U-A U C G G U C 160 U G~C 20 A ~ U C C A U U C 5:6 C 80 U A C C~G A-U A-U A~:~G ~:~ g:g U-A UUUAUGACACGCGUUCAAUUUAAACACCACCAUCAUC~G--------C~GUGGUGC~GAGA-UUUUUU ~l 40\ ~U hiS09654 60 100 180 +C his09876 A his09856 ilhis09613 C his09709 4:5 B A C A AUCAAAUGA G G~C U-A A-U 140 A U A A C~G G C A U U C 20 A G G U U A G U G U G~C U A A-U G~C~G A~U-A C~G MetrhrArgValGlnPheLysBisHisBisHisBisBisBisProAsp U-A C C~G his09864 U"G C~U-A C G~C G his09609 his09863 A A GA G~C his09693 AG G~C C~G U C G G A A U U 2:3 A~C~~C his09897 U-A his09896 his09713 G~U-A A~C~G~U his09712 U-A A-U UUUAUGACACGCGUUCAAUUUAAACACCACCAUCAUCACCAUCAUCCUGACUAG~CAUU----------CAG=CAUCUUCCGGGGGCUUUUUUUU 40 60 80 160 180 FIG. 4. Alternative secondary structures of the S ..typhimurium his leader transcript. Secondary structures 1:2,2:3,3:4,4:5, and 5:6 are those reported by Johnston and Roth (89). The positions of key mutations are indicated. (A) Termination configuration. (B) Antitermination configuration. region. Changing the AUG initiation codon to AUA (his09856; Fig. 4A) or ACG (his09709; Fig. 4A) caused a moderate reduction in his operon expression (weak phenotype; His+ AT'), whereas a deletion replacing the AUG with AG (his09613; Fig. 4A) blocked expression sufficiently to cause histidine auxotrophy. Not only do these results establish that leader peptide synthesis is required for readthrough at the his attenuator, they also suggest that the AUA and ACG codons may allow inefficient translation initiation since they only partially reduced his operon expression. Mutational changes in the sequence immediately preceding the his leader peptide start codon, which apparently sequester the ribosome-binding site in a hairpin secondary structure, can cause either a slight or large decrease in his operon expression, depending on the stability of the hairpin created. Mutations altering the leader peptide. Several non· sense mutations in the S. typhimurium his leader peptide coding region have been isolated (89). Analysis of these mutations suggests that the level of his operon expression is correlated with the position at which translation terminates in the leader peptide coding region. Mutations causing translation termination at codon positions 4 (his09675; Fig. 4A) or 5 (his09654; Fig. 4A) of the leader peptide coding region result in a strong His- phenotype, whereas an insertion mutation (his09876) causing termination at codon 7, immediately preceding the seven His codons, allows a modest increase in expression, leading to a weak His+ AT' phenotype. This result suggests that when the translating ribosome reaches codon position 7, but not codon position 5, it can occasionally disrupt structure 1 :2. Thus, the his attenuator has evolved so r , that the translating ribosome does not completely block formation of RNA structure 1:2 until it reaches the His codons beginning with codon 8 (Fig. 4A). When appropriate nonsense suppressors are combined with the his09654 (C ~ V, ochre) or his09675 (8-base-pair insertion, opal) alleles, the His- phenotype is suppressed (89). This result verifies that translation of the leader peptide coding region is required for normal his operon expression and also suggests that the exact amino acid sequence of the leader peptide is unimportant for some termination relief. When the his09675 allele is suppressed and translation continues out of frame to a VAA codon 30 bases past the normal leader peptide termination codon, transcription of the his operon is sufficient to give a His+ phenotype. This suggests that the translating ribosome closely follows RNA polymerase and disrupts structure 5:6 often enough to allow near-wildtype levels of transcription of the his operon structural genes. Finally, all hisO-type mutations that affect leader peptide synthesis are cis dominant. Taken together with the fact that the variant leader peptides produced by the his09876 allele and the suppressed his09675 allele allow at least some transcription to proceed through the attenuator region (His+ AT' phenotype), this result also suggests that the leader peptide per se has no function. Rather, it is the act of translation of the leader peptide coding region that is responsible for read through at the attenuator. Mutations affecting his leader transcript secondary structures. Analyses by Johnston and Roth (88, 89) of a set of mutants with alterations in the his leader region provided convincing evidence for the role of RNA secondary structures in governing attenuation. Changes that would weaken the predicted stabilities of either hairpin 2:3 (his09693, his09609, his09863; Fig. 4B) or hairpin 4:5 (his09864, his09896, his09713, his09897, his09712; Fig. 4B) cause greater termination. In general, those changes that would appreciably destabilize these hairpins (his09693, his09609; Fig. 4B) cause His auxotrophy, whereas those that would only slightly reduce their stability (his09863, his09864, his09896, his09713, his09897, his09712; Fig. 4B) produce the His+ AT' phenotype. These findings established that both hairpins 2:3 and 4:5 are required for anti terminator function in the his leader region. Johnston and Roth also characterized five deletions that removed portions of the antiterminator sequence (~108-118, ~109-122, ~116-120, ~116-124, ~156-159; see Fig. 4B). All five deletions cause a Hisphenotype. That changes in his anti terminator function are due to alterations in base pairing and not to primary sequence alterations was established by constructing the double mutant his09712 his09713 (M. Johnston and J. Roth, personal communication). The single mutant parents have a His+ AT' phenotype, indicating that hairpin 4:5 is weakened and hairpin 5:6 forms more readily. However, in the double mutant, the wild-type GC base pair is replaced by an AV base pair that partially restores the predicted stability of hairpin 4:5. The double mutant expresses wild-type levels of his enzymes. As predicted by the attenuation model, formation of a stable RNA secondary structure governs termination at the attenuator. 77 TRANSCRIPTION ATTENUATION 1285 Another interesting finding was that his mutations that affect anti terminator structure were suppressible by amber suppressors (86, 88, 89). All of these mutations are past the stop codon for the leader peptide and do not generate amber codons.1t is likely that the effect of these mutations is reversed by translation of the normal VAG leader peptide stop codon and continued ribosome movement to an in-frame VGA stop codon at the base of hairpin 5:6 (nucleotide 140, Fig. 4A). A ribosome approaching or positioned at the VGA stop codon apparently can block formation of 5:6 and allow sufficient transcription read through to overcome the His- phenotype. Mutations affecting tRNAHls synthesis. An interesting aspect of work on the histidine attenuator is that many of the mutations that increase expression of the his operon do so by affecting tRNA His or its synthesis (see chapter 25 for a more complete discussion of these mutations). In initial investigations with the his operon it was considered possible that a his repressor recognized tRNA His as its corepressor. Since no evidence for such a repressor protein ever emerged, alternative explanations were sought. Ames and coworkers (153) showed that the hisT mutation affects pseudouridine formation in several tRNAs, including tRNA His . Whereas hisT mutants possess wild-type levels of charged tRNA His (108) and synthesize 13galactosidase at a normal rate (86), they utilize supE (glutamine-inserting amber suppressor) to suppress the lacU281(Am) mutations less than 5% as well as wild type (86). These results suggest that increased expression of the his operon in hisT mutants is caused by inefficient translation of the seven His codons in the leader peptide coding region, thus allowing more frequent formation of the anti terminator RNA structure. Mutations affecting hisR, the tRNA His structural gene, can have a similar effect, or, like mutations that affect His-tRNA synthetase (hisS), they can affect leader peptide synthesis by reducing the efficiency of tRNA His charging, which would decrease the intracellular concentration of histidyl-tRNA His . Recently, Ames and co-workers (4) have pointed out the existence of a high degree of sequence (45 of 75 nucleotides) and structural homology between the his leader transcript and tRNAHis. They suggest that proteins known to interact with tRNA His , such as tRNA modifying and processing enzymes, histidyl-tRNA synthetase, the hisG enzyme, ribosomal proteins, and elongation factors, may interact with similar sites in his leader RNA. Such RNA-protein interactions could provide regulatory input and affect expression of the his operon by altering the efficiency of termination at the his attenuator. If such interactions occur, they not only would provide alternative explanations for the phenotypes discussed above but also would suggest that attenuation control of the his operon is considerably more complex than currently believed. Evidence for these possibilities, such as demonstrating modified bases in the his leader transcript or documenting a leader RNA-protein interaction, has not yet appeared. If this tRNA-leader RNA homology is indicative of additional interactions and events, it could provide fertile material for future studies on attenuation. Alternatively, the tRNAHiS-his leader RNA homology may reflect an evolutionary relationship, and leader 1286 LANDICK AND YANOFSKY RNA may be incapable of interacting with proteins that recognize tRNA (see Conclusions, below). The leu Operon Attenuation control of the leu operons of E. coli and S. typhimurium has been elucidated by Calvo and co-workers (26, 64, 92,150,172). In these organisms, leu enzyme levels are elevated as much as 50- and 40-fold, respectively, under conditions of leucine-limited growth (24). In both organisms, the levels of total leu operon mRNA parallel leu enzyme levels; in S. typhimurium it has been shown that leu leader RNA is synthesized constitutively, whereas the level of leu structural gene mRNA is regulated by exogenous leucine (150). All transcription regulation of the leu operon appears to be accomplished by attenuation; no evidence for a leu repressor has been obtained. The constitutivity of leu leader RNA synthesis (150) argues against repression. DNA sequence analyses and in vitro transcription studies suggest that leu leader RNA and trp leader RNA fold in similar secondary structures (Fig. SA); both the E. coli and S. typhimurium leu leader transcripts encode 28-residue peptides that contain 4 contiguous leucine residues. Comparison of the S. typhimurium and E. coli leu leader sequences reveals considerable conservation in the regions thought to be involved in RNA secondary structure formation, as well as the Leu codon regions. Regions of the leader thought not to be directly involved in attenuation show greater divergence. It is particularly interesting that, as in the case of the trp leader peptide, some leu leader peptide residues in the two organisms differCat codons 2, 8, 16, 17, 18,22, and 24; Fig. SA), in agreement with the notion that the leader peptide amino acid sequence per se (other than the Leu residues) is unimportant. Two different G ~ A changes in the 3:4 (terminator) hairpin of the S. typhimurium leu leader have been obtained (leu-2007 and leu-2009; Fig. SA; 24,25, 150). leu-2007 reduces the predicted stability of the hairpin from -24 to -14 Kcallmol (ca. -100 to -59 kJ/mol) and increases read through at the attenuator from 2 to 25% in an in vitro transcription system. Strains carrying the leu-200l allele have 30-fold higher levels of Jj-isopropylmalate dehydrogenase (leuB gene product) in excess Leu, but near wild-type levels under Leu starvation conditions, as would be expected on the basis of the attenuation model. Mutation leu-2009 reduces the predicted stability of the hairpin to -12 kcallmol (ca. 50 kJ/mol); its effect on leu operon expression is identical to that of leu-2007 (24, 25). Recently, Calvo and co-workers used site-specific mutagenesis to replace the four leader peptide Leu codons with four Thr codons (26). The regulation of this altered leu operon has some interesting implications for the role of control co dons in attenuation. As expected, the mutant strain did not show the 40-fold wild-type response to leucine limitation; however, it did exhibit a 3-fold increase in the Jj-isopropylmalate dehydrogenase level upon leucine limitation. More importantly, growth of a temperature-sensitive threonyl-tRNA synthetase mutant (containing the Leu ~ Thr changes) at the nonpermissive temperature caused a lO-fold increase in Jj-isopropylmalate dehydrogenase (26). These results argue that ribosomes stall less frequently at the Thr codons of the mutant leader transcript during threonine limitation than at the wild-type Leu codons during leucine limitation. CUA, the Leu codon used predominantly, is a rarely used Leu codon (66) whose cognate tRNA is present at a low concentration (29), whereas the ACU Thr co dons that replace the Leu codons are translated efficiently because their cognate tRNA is abundant (29). Thus leucine limitation may stall ribosomes more efficiently at CUA codons than threonine limitation reduces ribosome movement over ACU codons. Calvo and co-workers suggested that accumulation of undermodified tRNAs may cause the residual response of the mutant to leucine limitation. Undermodified tRNAs do accumulate during leucine starvation (95). As suggested for the effect of hisT on his operon attenuation (86), undermodified tRNAs may slow translation, allowing some ribosomes to reside in critical regions of the leader peptide coding region long enough to promote antiterminator formation. The response of the wild-type leu operon to threonine limitation may have the same explanation. Both of these arguments (codon translatability and tRNA undermodification) suggest that attenuation could be fine-tuned by adjusting codon usage in a leader peptide coding region (see Leader Peptide Sequences and Functions, below). The thr Operon The threonine (thr) operon of E. coli encodes three polypeptides; these polypeptides are responsible for four of the five enzymatic activities required for synthesis of threonine from aspartic acid (54, 166). The first gene in the operon, thrA, specifies a bifunctional enzyme, aspartokinase I-homoserine dehydrogenase I, which catalyzes reactions 1 and 3 of the common pathway that leads to the formation of threonine, diaminopimelic acid, lysine, methionine, and isoleucine. The other two genes of the operon, thrB and thrC, encode polypeptides that catalyze steps 4 and 5 of the specific pathway leading to threonine. In both S. typhimurium and E. coli, limiting the growth of appropriate auxotrophs by withholding either threonine or isoleucine causes a 20-fold increase in the level of the thrA polypeptide; thus the thr operon is regulated by both threonine and isoleucine. In this respect, the thr operon differs from the three operons discussed so far, the trp, his, and leu operons. It initially was thought to be regulated by "multivalent repression" (54); however, much as in the history of his operon regulation, what was initially considered to be regulation by repression turned out to be regulation by transcription attenuation. Gardner (61) reported the DNA sequence of the region immediately preceding thrA. This region contained both a coding region for a 21-amino acid leader peptide, with 4 lIe and 8 Thr residues, and segments that could specify potential alternative RNA secondary structures (Fig. 5B) similar to those observed in the trp and leu operons. Transcription begins 189 nucleotides preceding the AUG start codon of thrA (61). During in vitro transcription, RNA polymerase pauses after synthesis of the 1:2 RNA hairpin (61), and 90% of transcripts terminate immediately after the 3:4 hairpin structure (114; Fig. 5B). These findings are 77 1287 TRANSCRIPTION ATTENUATION 1:2 A U A U Phe U I1. U U G A-U Ala C=G Asn G=C C=G Val Arg CAA Leu 2:3 CU AU G C Leu GAU Leu C=G A Gly Stop Arg G=C U G G A Ph. Arg G U G A A 3:4 Val C Gil} U'G U-A U-A U C=G G=C 140 C"'G G=C C=G_A leuL2007 U Val C Ile G U C=G 20 C AA CA AA Gln U'G AUAACGCAUUCGC ,I.e His Pro C=G A-U C=G U'G U U'G G=C G U C U G The AA A A G=C Gil} 120 CG AG U-A C=G Leu U uC A-U A A C C Gl Y G=C Gly G=C C=G G=C G=C G=C C=G Gly G=C Ile Tie C NetSerHisU-A GlnBisStop 140 G C=G - - A leuL2009 A-U Val Pro Leu LeuLe uAsnAl a PheI 1 eVal ArgGl yArg A-U A-U U"G G- U G' U C U AC U AC UC MC UCAU U UAUUG UGGGCGGUAGACG =GC GCGGGU UU UU U U 60 80 160 UUAAU GUCAC A UA- U UCAAC AU U AAGUCAGC UCGAAG UCAAACA- UU UU 120 160 B GCAUAGCGCACAG A C 20 U A 2:3 A U U A 3:4 A 120 G A AG AC "C AC G A C U C A C C=G A-U thrL140 A_C=G __ A.U thrLISl thrLIJ9 A +--G=C 1:2 Thr C A Thr C=G C=G A-U A A U G GG G AA CA Stop AA G A U-A G1 Y C:=G-+-U thrL135 G1 Y G=C G=C G::C--+-A thrLl]8 Ala C::G--....A thrLIJ9 G=C____.._A thrL140 G1!l U-A ThrThrGl yA.nG"C ACC ACA GGU AAC G =C UGAC AG UGG GGGC U U U UU U U U 160 consistent with the general attenuation model discussed earlier. Segments of the thr leader RNA resistant to RNase T, have been identified (61); the data obtained agree with the structures shown in Fig. SB. However, the exact structure assumed by folded thr leader RNA is as yet unknown. Secondary structure predictions us- ing the method of Zuker (185) suggest more extensive 1:2 and 2:3 structures than pictured in Fig. SB (see reference 96). Lynn et al. (113) have proposed two alternative forms of the 1:2 hairpin structure: the one shown in Fig. SB, and a second that pairs RNA segments 78 to 87 with RNA segment 2. Interestingly, RNA segments 60 to 69 and 78 to 87, termed lA and IB by 1288 LAN DICK AND YANOFSKY 2:3 G C A A A C G Stop AI. 1:2 A Val CCUG Gl Y 3:4 A G A G stop AGCAUCUU G Leu C=G Gly U CA ACe U-A U-A I 120 GA Uc CA G=C Ala G=C C G:::C 160 C=G 'thr ~ A CG Ala A-U AG Arg Val 20 A C C:G U A G A A Leu C:::G Ala C=G C",G Ala C=G AI. G:::C 140 C=G G=C Ala LlJs G=C C:G "et A-U LeuGIYArgGl yA-U C=G AUUCCUUCGAACAAGAUGCAAGAAA AGAC AAAAUGAC =GC AC U UGGA GAGGAA- UAACGAAC UAAGA- UUUU UUU U IIe CG G Uu Leu G A C:G GUCGU GAUUA UG A AC G=C Ser G A Uu A GG Ser G·U Ile C::G CIj_ Leu 140 G" Arg G=C G=C Pro U-A G=C G=C Pro U·G V.l A. Gl Y IIo U Val Val Ly. U U-A A-U G=C V.l IIo U U IIo U U-A U-A C-G G=C G-C A-U A-U Gl!l GaC G=C 160 C-G eys G=C Pro U-A Pro G"'C valValValIleIleIle C C GUGGU GG UGA U U AUU A UCCC A C = GA A AGGUC C GGGGGU U UU U U UU 80 180 180 P o 1:2 U A C C Pro Ser C C:G 80 G=C AI. C CoG G=C U-A CoG AI. Sor Ala v., A A-U CoG CoG Pro A-lJ Leu U Val C CoG A-U Leu ell LYB Val c _p A II" G A-U C::<G AI. Val G=C C:G Arg A-U Asn 100 UCA Leu C v., GU Met Ser A-U CoG C=G Thr Val CoG A-U U·G C-G v., A-U Val U'G A-U Val Gl!l Pro stop Aro G U Val G U A-U A-U G=C G G=C U-A G CoG U·G G=C 160 StopG=C GoU Val G-C GU AC AA CA G-C C=G U·G v.l C A GoU Val v.l A G U C G-C G ProCzG U Val CaG G-C G-C Ala C .. G Gly AlaeaG Ly_LeuLeuproSerAlaProSerC"'G A_n Gee AAACU A CUACC AAC UGC GCC A UCCG' C A - - - - - - AU- AAACCGGGCGGGGU UUU UCGU U U 60 140 G U Val v.l AI. C'G A-U A-U U-A A- U AU UGU UAA"C AC II A A ACe II ACAAGG =C UGGA A C"AC "C"eGA UUC CA- U UCG UU U 20 V.l A G C C A A A G=C G=C G CoG CoG G=C CoG 160 CoG 180 CoG G C A.sn A 100 U 3:4 CGGCAAU GCCGCG U Ala C=G CoG C-G C A G'C Ifet C A C-G U G 40 U C 80 180 FIG. 5. Continued Gardner, are direct repeats. Each contains two of the eight Thr codons and one of the four lIe codons for the leader peptide. The presence of these repeats probably increases the regulatory capacity of the thr leader region. It is unclear which, if either, of the two potential 1:2 hairpins actually forms in the thr leader transcript. The regulatory mutation thr-79-20, isolated on the basis of increased thr operon expression (63), inserts a C residue in segment 4 of the terminator structure (61; Fig. 5B) and reduces the efficiency of in vitro transcription termination from 90 to 75% (114). A complete deletion of the 3:4 hairpin, thr01028 (nucleotides 107 to ISS, Fig. 5B), was isolated using a protein fusion of thrA to lacZ (147). This deletion caused a lO-fold increase in r3-galactosidase activity as compared with lacZ fusions that retain the entire leader region (130). Recently, Gardner and co-workers have characterized 10 additional mutational changes in the 77 TRANSCRIPTION ATTENUATION 1289 1:2 E ~ Stop A G U U G C=G G~AUG C=G Pro A CA C=G C=G GA U GU 80 Phe C GU Uu AG GC U U·G 3:4 U-A U-A ph. U C U-A C=G Phe U-~ U-A ~-U C=G G=C C=G Ala 100 120 ~ U ~ C C=G C=G C=G U·G C=G C=G G=C U-A U-A G:::C A-U 8etLysBl_IleProPhe C=G A-U Phe Phe 40 A A A A-U Stop G=C U·G 140 AAGUCACUUAAGGAAACAA~CAUGAAACACAUACCGUUUUU-ACGAACAAUA-UUUU 20 U U-A U-A 80 U-A G G C=G 120 G=C G=C A-U G=C G=C G=C U-A C=G U-~ AA C GC ~-U ph. CA UG C UG C Thr A~ GA C 60 CG AA Pro C=G Phe . C=G 8etLysHisIlePcoPhePhePheAlaPhePbePherbr C=G AUGAAACACAUACCGUUUUUCUUCGCAUUCUUUUUUAACUUC=GGCCUUUUUU 40 60 140 1:2 F 80 A U A C G=C U-A C=G C=G Stop Thr A Ser Phe A C=GG G=C A-U U-A U·G 2:3 C U·G C Tyr phe UCUUUU Phe C=G A-U U·G U-A U·G U-A U-A U·G U-A C=G U-A 100 40 C G G G A G A A A A 100 A G A A Phe G A G Arg G=C AG U C=G C U A C=G C=G A Ph. U-A C=G 140 C U-A U-A C U-A C=G A IIe U-A C=G A A C G=C A AI. U-A A-U A Neta.nAl.C=G A-U GGUAACGCAAGCAAUGAAUGCUG=CGCCUGA-UUUUU CU-\ 20 A A G A A G=C U-A G C=G G-C C=G G=C 3:4 120 Cu A G AA U-A CoG G=C G-C A-U PheArgPbePhePheTyrPheSerrhr G=C uUCCGCUUCUUUUUUUACUUUAGCACCUGAAUCCAG=CCAGUGGAGGCUUUUUUU 120 60 80 140 FIG. 5. Continued 3:4 hairpin that increase homoserine dehydrogenase levels from 2- to lO-fold when the corresponding mutant strains are grown in excess threonine and isoleucine (113). In general. the predicted stabilities of the altered RNA terminator structures correlate with their termination efficiency. However, several exceptions to this conclusion were noted. Strains with thrL135 U, thrL139 A, and thrL156 U mutations (mutations are designated by the leader position at which the substitution occurs, followed by the nucleotide present in the mutant) (Fig. SB) gave only modest increases in the level of homoserine dehydrogenase (approximately twofold) even though the calculated stabilities of the 3:4 RNA secondary structures were similar to those of mutants showing a 5- to lO-fold increase in homo serine dehydrogenase. A strain with the thr-79-20 mutation synthesized high levels of homoserine dehydrogenase even though the calculated stability of its 3:4 hairpin was only slightly lower than that of wild type. Lynn et al. (113) suggested that 1290 LAN DICK AND YANOFSKY a specific hairpin structure may be required for transcription termination. Mutations that cause large increases in homoserine dehydrogenase expression might alter this structure substantially, whereas mutations that cause only modest increases in expression might leave the key portions of this structure unchanged even though they weaken its predicted stability. Alternatively, the findings of Lynn et al. may reflect our incomplete understanding of all the features of RNA secondary structures that influence stability. Site-specific mutagenesis has been used to replace the thr leader peptide stop codon by UGG (Trp); this change extends the leader peptide coding region to a UGA stop codon in the loop of the 3:4 secondary structure (Fig. 5B; 141). A thrA-IacZ fusion was used to monitor the effects of this change. The twofold reduction in f3-galactosidase levels observed in wild-type cells when excess isoleucine and valine were present was almost eliminated in the UGG mutant. These results corroborate the findings of Johnston and Roth with the his operon (86, 88, 89). In both cases, translation through the terminator leads to increased readthrough at the attenuator. The ilvGMEDA Operon The ilvGMEDA operons of E. coli and S. typhimurium encode four enzymes involved in isoleucine and valine biosynthesis: acetohydroxy acid synthetase II (valine resistant; ilvG and ilvM), branched-chain amino acid aminotransferase (ilvE), dihydroxy acid dehydratase (ilvD), and threonine deaminase (ilvA). In conjunction with polypeptides encoded by other ilv genes, these enzymes catalyze the conversion of threonine to isoleucine via condensation of threonine deaminase-derived a-ketobutyrate and pyruvate and the synthesis of valine from pyruvate. The pathways of isoleucine and valine synthesis are parallel for four analogous steps beginning with the condensation of either two pyruvate molecules or one molecule each of pyruvate and a-ketobutyrate. In E. coli K-12, the ilvG gene is normally cryptic because of a frameshift mutation within the ilvG reading frame (102). This mutation renders E. coli K-12 susceptible to growth inhibition by valine owing to the valine sensitivity of the acetohydroxy acid synthetase I encoded by the ilvE gene. Other strains of E. coli and S. typhimurium encode a normal ilvG gene and are valine resistant. ilvM, the recently discovered fifth gene in the ilvGEDA operon (M.-F. Lu and H. E. Umbarger, Fed. Proc. 44:1417,1985), apparently encodes the small subunit of acetohydroxy acid synthetase II (R. M. Kutny and J. V. Schoss, Fed. Proc. 43:1867, 1984). Deletion of ilvM resulted in loss of acetohydroxy acid synthetase II activity in E. coli (Lu and Umbarger, Fed. Proc. 44:1417,1985). The ilvGMEDA operon is regulated multivalently by the levels of aminoacylated tRNA Val, tRNA Ilc , and tRNALeu (20, 154). Multivalent control of ilvGMEDA transcription is mediated by attenuation and involves synthesis of a 32-residue peptide containing six valine, five isoleucine, and five leucine residues (103). The leader region is 270 nucleotides in length (1), and it yields a 185-nucleotide terminated transcript that potentially can fold into secondary structures (Fig. 5C) similar to those involved in other examples of atten- uation. A transcription pause site in the ilvGMEDA leader region has been documented at position 117 (Fig. 5C) shortly after the 1:2 hairpin (73). This site presumably functions to synchronize transcription and leader peptide synthesis as postulated for the trp operon (see Roles of the Transcript Pause and Antiterminator Secondary Structures, above). One distinguishing feature of the ilvGMEDA transcript is the bifurcated stem-loop structure that is postulated to serve as the 1:2 hairpin (Fig. 5C). Interestingly, a complex 1:2 stem-loop also has been predicted for the ilvEN attenuator (see The ilvEN Operon, below). To explain the participation of these complicated structures in attenuation, it has been suggested that two ribosomes may occupy segment 1 of ilv leader RNA during amino acid starvation. The two leucine co dons thought to be responsible for reduced termination in response to leucine starvation are located six (ilvGMEDA) or eight (ilvEN) codons preceding the Val control codons. In a static view of attenuation, a ribosome stalled at Valor Ile co dons at the top of structure 1:2 would not prevent base pairing at the bottom of 1 :2. A ribosome stalled at this location, then, might not promote formation of the antiterminator and hence would allow the terminator hairpin to form. To explain ilv attenuation, Hatfield and colleagues (71, 103) proposed that when a ribosome stalls at the top of 1 :2, a second ribosome initiates synthesis of the leader peptide on the same transcript, encounters the stalled ribosome, and blocks the bottom of 1 :2. This so-called "double-ribosome" model has been applied to regulation of both ilvGMEDA and ilvEN. To date there has not been any experimental test of this model. An alternative view is that once the initial translating ribosome has disrupted the 1:2 hairpin and stalled at the upper 1:2 portion, the presence of the newly synthesized distal sequences would favor formation of the antiterminator rather than the bottom of the 1:2 hairpin. Indeed, this seems to be a more plausible explanation, given current thinking on the role of translational release of transcription pausing in synchronizing leader region transcription and leader transcript translation in operons regulated by at· tenuation (see Roles of the Transcript Pause and Antiterminator Secondary Structures, above). If RNA polymerase pauses after synthesizing the first 117 nucleotides of the ilvGMEDA leader transcript (73; Fig. 5C) and this paused complex is released when the translating ribosome disrupts the 1:2 hairpin, it is difficult to imagine how a second ribosome could initiate translation and move to the appropriate location before the attenuation decision was made. The ilvBN Operon The ilvE gene of E. coli and S. typhimurium encodes the valine-sensitive acetohydroxy acid synthetase I described in the previous section. The size of the ilvB transcription unit is unknown; however, it apparently contains ilvN, the gene for the small subunit of this enzyme (56 171). In addition to regulation by ppGpp (55) and cyclic AMP (55,56, 165), transcription of the ilvEN gene is controlled by a transcription attenuator responsive to starvation for leucine and valine, the end products of the acetohydroxy acid synthetase I 77 pathway (56, 71). Thus ilvBN regulation is another example of multivalent attenuation. There is no control by isoleucine, consistent with the fact that acetohydroxy acid synthetase I is feedback inhibited only by valine. In vitro transcription of the ilvBN leader region gives a 188-nucleotide terminated leader transcript that encodes a 32-amino acid peptide containing nine valine and three leucine residues (71). The leader transcript potentially can form alternative secondary structures (Fig. 5D) that are consistent with an attenuation mechanism. Hauser and Hatfield (72) have proposed that ilvBN attenuation is controlled not only by leucine and valine, the pathway end products, but also by threonine and alanine, amino acids that are substrates in these pathways. Threonine is deaminated to o.-ketobutyrate by threonine deaminase, the ilvA gene product, and alanine is deaminated to pyruvate by alanine transaminase; o.-ketobutyrate and pyruvate are the substrates of acetohydroxy acid synthetase. This form of control has been termed substrate attenuation. Using hybridization probes to detect leader versus distal RNA, Hauser and Hatfield (72) observed attenuation relief in the ilvBN operon in response to Leu, Val, Thr, and Ala starvation. However, in a separate study (167), no significant elevation of acetohydroxy acid synthetase I levels was observed in response to Thr or Ala starvation. The explanation for this apparent disagreement is unknown. The pheA Operon The gene pheA encodes chorismate mutase-prephenate dehydratase, the enzyme that catalyzes the first two of the three steps in phenylalanine synthesis from chorismate. In E. coli, pheA is found in a monocistronic operon at map position 57 next to the oppositely transcribed aroF-tyrA operon (8, 76a). DNA sequence and in vitro transcription studies have revealed that the 170-base-pair pheA leader region has the features of a classic attenuation region (76a, 186). A 145-base terminated in vitro transcript has been identified (186) which contains a coding region for a IS-residue leader peptide with 7 phenylalanine residues; the leader transcript can fold into both terminator and anti terminator configurations (Fig. 5E). An alternative folding scheme in which the leader peptide coding region pairs with the loop region of an alternate 1:2 hairpin has also been described (186). Mutations in E. coli that alter RNA polymerase (rpoB rifampin resistance mutations) or block the modification of tRNA Phe (miaA) or affect a gene of unknown function that maps at min 93 (pheR) all lead to elevated expression of pheA (65). The rpoB and miaA effects agree with the proposed transcription attenuation mechanism, whereas the effect of pheR suggests that a separate mechanism also may control pheA expression. The features of the pheA leader region otherwise are unremarkable. LEADER PEPTIDE SEQUENCES AND FUNCTIONS The leader peptides of amino acid biosynthetic operons regulated by attenuation generally contain TRANSCRIPTION ATTENUATION 1291 multiple residues of the regulating amino acid(s). As we have seen, the ability or inability to incorporate these amino acids during leader peptide synthesis provides the basis for the decision whether or not to terminate transcription at the attenuator. Comparison of leader peptide coding regions reveals considerable variation in the number and positions of regulatory codons. Codon usage varies as well. In addition, each peptide coding region contains codons for amino acids that have no apparent relationship to regulating amino acids. These features raise fundamental questions about the regulatory significance of leader peptides and their coding regions. The leader peptides themselves appear to play no regulatory role; all tests for a trans-acting function have been negative. In addition, the existing data suggest that leader peptides are degraded rapidly. These observations are consistent with the conclusion from the many studies on attenuation, namely, that it is the act of leader peptide synthesis that has regulatory significance. The number and distribution of regulatory codons varies from operon to operon and from species to species. We suspect that this variation reflects fundamental differences in the extent to which the different operons are regulated by attenuation. Thus in the trp operon, where attenuation allows only modest regulation (179) and then only under conditions of severe tryptophan starvation, there are only two Trp codons in the leader peptide coding region. By contrast, in the His operon, where attenuation has greater regulatory importance, there are seven His codons in the leader peptide coding region. A significant difference in regulatory codon number also is seen for Leu codons in the ilvGMEDA leader regions of E. coli versus S. marcescens: there are four Leu codons in the former but only one in the latter (70). Either the single Leu codon of S. marcescens is functionally equivalent to the four Leu codons of E. coli (76), or leucine regulation of the ilvGMEDA operon is of lesser importance in S. marcescens. Rare synonymous codons are present in the leader peptide coding regions of several operons regulated by transcription attenuation. For example, in the leu leader peptide coding region of S. typhimurium three of the four Leu codons are CVA, whereas in the ilvGMEDA leader peptide coding region of S. marcescens the sole Leu codon used is CVA. The use of rare co dons in leader peptide coding regions could increase the cell's sensitivity to amino acid starvation. The minor isoaccepting tRNAs that correspond to rare co dons may be charged poorly or may translate inefficiently under starvation conditions; thus, attenuation would be relieved more readily upon amino acid starvation. Regulatory co dons often are interspersed with other codons, as in the leader regions of the phe and ilv operons. There may be several explanations for such arrangements, but certainly location and spacing of regulatory co dons are most relevant to the locations of the RNA segments that form regulatory secondary structures. The significance of co dons for apparently unrelated amino acids in leader peptide coding regions is not obvious. Starvation for these amino acids often can relieve termination when the corresponding codons are located adjacent to a regulatory codon. In one 1292 LANDICK AND YANOFSKY such instance, in which Ala limitation relieves termination in the ilvBN operon, there is a relationship between the Ala and Ilv metabolic pathways (72). However, as mentioned, this finding has been questioned (167). With regard to the trp operon there are no obvious relationships between the Arg, His, and Trp biosynthetic pathways that would account for the relief of attenuation that accompanies Arg or His starvation (164). One possible explanation for the existence of presumably unrelated codons in leader peptide coding regions is their use to signal whether the cell has many of the amino acids needed for protein synthesis. Thus it has been shown that mutations that limit initiation of synthesis of the trp or his leader peptides increase transcription termination at the respective attenuator (88, 89, 163). Conceivably, ribosome stalling over one of the initial co dons in the corresponding peptide coding region can have the same effect. TRANSCRIPTION ATTENUATION IN NON-AMINO ACID BIOSYNTHETIC OPERONS Following elucidation of the mechanism of attenuation control of amino acid biosynthetic operons, many workers expected that variations on this regulatory mechanism would be found to control expression of genes for other cellular functions (e.g., catabolism of amino acids, amino acid transport, and synthesis of other metabolic products). In fact, transcription attenuators have been demonstrated or implicated for many such operons, but the diversity of attenuation mechanisms that has been observed was unanticipated. In contrast to the uniformity that is apparent from studies of attenuation in amino acid biosynthetic operons, these mechanisms appear to reflect accommodation to the regulatory peculiarities of each function or operon. Next we shall discuss several examples demonstrating this diversity. The pheST Operon The pheST operon of E. coli encodes the small (pheS) and large (pheT) subunits of phenylalanyl-tRNA synthetase (30, 116). Unlike expression of the genes for alanyl-tRNA synthetase (alaS), which is subject to autogenous transcriptional repression (137), and the gene for threonyl-tRNA synthetase (thrS) , which is con trolled by translational repression (157), pheST is regulated by transcription attenuation (156, 158). In vitro transcription of the pheST leader region produces transcripts initiating 351 base pairs preceding the pheS AUG codon (48). Most of these transcripts terminate in the leader region at a site 150 base pairs downstream from the initiation site; termination at this site was relieved by substituting ITP for GTP in the transcription reaction (48). The nucleotide sequence of the pheST leader region revealed a classic arrangement of potential alternative secondary structures in the first 150 bases of the pheST leader transcript and an open reading frame for a 14-residue leader peptide containing five phenylalanine residues (Fig. SF). Recent studies have shown that deletion of the terminator structure (3:4, Fig. SF) leads to a lO-fold increase in transcription of downstream sequences and that undermodification of phenylalanyl- tRNA caused by the miaA mutation increases expression of the operon as well (156). These results indicate that ph eST is regulated much like the amino acid biosynthetic operons regulated by attenuation. In contrast to attenuation in amino acid biosynthetic operons, however, more than 200 base pairs separate the attenuator from the pheS start codon. The function of this segment, which includes a 31-codon open reading frame with no phenylalanine codons, is unknown. Translation of this reading frame might well be required to prevent Rho-dependent termination in the 200-base-pair spacer region. Possible roles of the spacer region and the second leader peptide are important unsolved problems. Pyrimidine Biosynthetic Operons In E. coli and S. typhimurium, UMP is synthesized from aspartate and carbamoylphosphate in five enzymatically catalyzed steps (26). The enzymes for these conversions are encoded in five unlinked operons: pyrB!, for the catalytic and regulatory subunits of aspartate transcarbamoylase; pyrc, for dihydroorotase; pyrD, for dihydroorotate oxidase; pyrE, for oro tate phosphoribosyltransferase; and pyrF, for orotidine-5' -monophosphate decarboxylase. Carbamoylphosphate is synthesized from ATP, CO 2 , and glutamine by carbamoylphosphate synthetase, the product of the carAB operons (designated pyrA in S. typhimurium). Pyrimidine starvation results in increased rates of synthesis of all six of these enzymes (93, 131, 149). The response of the pyr genes is noncoordinate, suggesting that individualized regulatory controls exist for each operon. The most thoroughly characterized pyr regulatory system is that of the pyrB! operon. Expression of this operon is regulated transcriptionally by the intracellular concentration of UTP and involves UTP-dependent transcription attenuation (168). The proposed regulatory mechanism is substantially different from the attenuation mechanism used to control amino acid biosynthetic operons. In vivo, most pyrB! transcripts originate approximately 160 nucleotides preceding the pyrB start codon (122). In vitro, a promoter 350 nucleotides upstream from this start codon also is utilized; transcripts initiating at both promoters usually terminate in a region of dyad symmetry centered 40 base pairs before the start of pyrB (3:4 in Fig. 6). The RNA terminator encoded by this symmetrical region is typical of those found in amino acid biosynthetic operons; it contains a GC-rich hairpin followed by a run of U residues. However, there is no apparent alternative RNA secondary structure that could prevent formation of the pyrB! terminator. There is a second hairpin that can form in the leader transcript; it is centered about 100 bases preceding the pyrB AUG codon (1:2 in Fig. 6A). RNA polymerase transcribing the pyrB! leader region in vitro pauses after synthesis of this hairpin 0:2 in Fig. 6) in a UTP-dependent process (168). The pyrB! leader transcript encodes a 44-residue leader peptide whose coding region spans both the 1:2 and 3:4 RNA hairpins (Fig. 6). These features have been incorporated into a model for pyrB! attenuation that can account for the increased expression associated with uridine starvation 77 TRANSCRIPTION ATTENUATION 1293 ACAAU U U G Lys C C 60 A A A G G G A G G A Leu Arg U G A G A U-A C=G U-A G=C C=G G=C Pro Ne~ValGlnCysValArgBisPheValLeuC=G Asn A U Lys A C 120 C=G Arg Leu U-A C=G C=G Gly Pro C=G GI n C =G Al a Asp Ala U GI y LeuProPhePhePheProLeuIleThrHisSer G=CPhePhe UAUGGUUCAGUGUGUUCGACAUUUUGUCUUAC=GCCUGCCGUUUUUCUUCCCGUUGAUCACCCAUUCCCA-UUUUUUUU 20 40 ~ 100 P FIG. 6. Secondary structures in the E. coli pyrBlleader transcript. Region of transcription pausing (168) is indicated by the bracket. (122, 143, 168; Fig. 7). According to this model, transcription attenuation in the pyrB! leader region is controlled by the rate of release of the transcription pause complex, formed after synthesis of RNA structure 1 :2. It is assumed that when the cell has an adequate pyrimidine supply, the resulting high UTP pool allows the paused transcription complex to release before a ribosome can initiate leader peptide synthesis and catch up with the transcribing RNA polymerase molecule (Fig. 7). Once polymerase escapes the pause site and it is not closely followed by a translating ribosome, it can rapidly elongate the message and synthesize the terminator (3:4 hairpin, Fig. 6), which will cause transcription termination. However, when a cell is starved for pyrimidines and has a low UTP pool, the paused transcription complex may remain at the pause site until it is approached by the ribosome synthesizing the leader peptide (Fig. 7). The ribosome could release the paused transcription complex in a manner analogous to pause complex release in the trp operon leader region, allowing the transcribing polymerase molecule and the translating ribosome to proceed in a tightly coupled fashion. As RNA segments 3 and 4 are synthesized, the translating ribosome could prevent segment 3 from base pairing with segment 4. If the RNA terminator does not form, transcription will proceed into the pyrB! structural gene region. The overall result is that pyrimidine starvation promotes transcription readthrough at the pyrB! attenuator. Thus, transcription pausing, exaggerated and controlled by pyrimidine limitation, and translation of a LOW UTP POLYMERASE PAUSES ~ PPI' HIGH UTP POLYMERASE DOESN'T PAUSE I2 ~" ppp/AUG : 1·2 j RIBOSOME INITIATES TRANSLATION AND RELEASES PAUSED POL YMERASE ~ ", RIBOSOME LAGS BEHIND POLYMERASE GAC PPP--AUG RIBOSOME DISRUPTS TERMINATOR TERMINATOR FORMS READ - THROUGH TERMINATION FIG. 7. Model for pyrBl attenuation (168). A ribosome is able to initiate translation and cause transcription readthrough only when RNA polymerase pauses in response to UTP limitation. 1294 LAN DICK AND YANOFSKY leader peptide coding region allow efficient regulation of transcription termination in the pyrBI operon. This model has the intriguing consequence that termination would occur whenever the cell is unable to synthesize the pyrBI leader peptide efficiently. These effects would protect the cell from wasting energy and nucleotides on pyrBI mRNA synthesis when it is incapable of synthesizing proteins. This result is reminiscent of superattenuation in amino acid biosynthetic operons (see Leader Peptide Sequences and Functions, above). There is much experimental support for transcription attenuation control of pyrBI expression. An S. typhimurium mutant with an altered RNA polymerase constitutively expresses elevated levels of aspartate transcarbamoylase and orotate phosphoribosyltransferase (84). In this mutant, RNA polymerase pausing at the pyrBI pause site may not be relieved by elevated UTP levels. Levin and Schachman (107) and Roland et al. (142) have coupled the pyrBI leader region to galK and lacZ, respectively, and have found that galactokinase and ~-galactosidase levels are increased in response to uracil starvation. When deletions removing the 3:4 hairpin region (Fig. 6) were introduced, uracil-dependent regulation was reduced appreciably (107, 142). Translation initiation at the pyrBI leader peptide initiation codon did occur when the leader peptide coding region was fused to lacZ (27, 142). When the pyrBI leader peptide initiation codon was changed from AUG to ACG, expression and regulation of the pyrBI operon was reduced greatly (142). Using a pyrB-IacZ fusion, uracil-dependent regulation was observed regardless of the reading frame in which ribosomes translated the 3:4 hairpin region, but was lost when translation terminated prematurely at a stop codon 49 nucleotides before the 3:4 hairpin (27). The pyrBI system offers perhaps the best opportunity to examine the in vivo role of pausing in attenuation. It would be particularly interesting to isolate pause RNA from UTP-starved cells and demonstrate the expected decrease in pause RNA stability when the UTP supply was adequate. Another objective should be to replace the run of U residues immediately following the 1:2 hairpin. If the model is correct, this should abolish UTP-sensitive pausing and prevent termination relief. A similar but less well substantiated model has been proposed for attenuation control of transcription of pyrE, the structural gene for orotate phosphoribosyltransferase (19, 136). The pyrE "leader transcript" originates over 800 nucleotides preceding pyrE and contains an open reading frame (designated orfE) that encodes a polypeptide 238 residues in length. orfE is separated from the pyrE coding region by sequences capable of forming an RNA hairpin typical of Rho-independent terminators. A 238-residue leader peptide is unprecedented; it is likely that orfE encodes a protein with a catalytic or regulatory function. Although it was shown in vitro that transcription of a DNA fragment containing pyrE terminated at a site corresponding to the potential RNA hairpin between orfE and the pyrE start codon (136), no UTP-dependent pause site has been identified. Bonekamp et al. (19) have identified elements in the pyrE leader region essential for what appears to be attenuation control. They constructed a plasmid that contains the lac promoter-operator and the first few co dons of lacZ, fused to the last 14 codons of orfE preceding the presumptive pyrE attenuator. This plasmid also has an in-frame fusion of pyrE to lacZ. By changing the lacZ-orfE fusion, they varied the position of the translation stop for orfE. UTP-sensitive regulation occurred when translation could proceed to the terminator or end 8 nucleotides before it (natural arrangement), but was lost when translation stopped 31 or 62 nucleotides before the terminator. These results suggest that UTP-dependent regulation of pyrE attenuation requires that translation of the upstream open reading frame proceed to the terminator segment of the transcript. However, they do not address the mechanism of translation-transcription coupling, nor do they suggest how UTP depletion might relieve attenuation. While the evidence for a pyrE attenuator is persuasive, key questions remain unanswered. In particular, experiments to determine the function of the 238-residue protein encoded by orfE and to detect UTP-dependent transcription pausing in the leader region are desirable. Analyses of pyrA, pyrc, pyrD, and pyrF regulation also are attractive goals for future investigations. The ampC Operon The ampC gene of E. coli encodes a ~-lactamase that is normally produced in small amounts and secreted into the periplasm, where it hydrolyzes penicillin and related antibiotics (125). Expression of ampC is not induced by members of this family of antibiotics; however, its expression is growth rate regulated (83). The transcript of the ampC operon begins 41 base pairs preceding the ampC structural gene. Transcription of the ampC leader region in vitro generates a terminated transcript ending at a typical Rho-independent terminator. Mutational analyses have shown that a mutation that introduces a base-pair mismatch in the terminator eliminates growth rate regulation (82). This mutation also increases readthrough transcription in vitro. Interestingly, the 41-nucleotide ampC leader RNA does not contain sequences that might form an alternative secondary structure that could preclude terminator formation, nor does it contain a peptide coding region. However, it does contain tandem translation start (AUG) and stop (UAA) co dons beginning at position 8 of the leader transcript (82), but no discernible Shine-Dalgarno sequence. To explain growth rate regulation, Normark and co-workers proposed that the first 10 residues of the leader transcript serve as a weak ribosome-binding site and that a ribosome bound at this site prevents formation of the terminator (82). Since rapidly growing cells have a higher ribosome content than slowly growing cells (78), rapid growth would increase ribosome binding at this site and promote read through into the ampC gene. While this ingenious model can account for growth rate-dependent attenuation of ampC transcription, it raises several unanswered questions. Can a ribosome bind at the (apparently) weak ampC leader ribosome-binding site fast enough to prevent terminator formation? Also, is there a significant increase in the number of free ribosomes in rapidly growing cells? TRANSCRIPTION ATTENUATION 77 rRNA Operons A Pl In E. coli and S. typhimurium the 16S, 23S, and 5S rRNAs are encoded in seven unlinked operons approximately 5,000 base pairs in length (43). All seven operons are organized similarly and have the order 165, 23S, 5S. Genes for tRNA are located between the 165 and 23S rRNA genes in these operons and after the 55 gene in some of the operons. In polypeptidespecifying operons, transcription generally terminates prematurely when translation is prevented, apparently because the absence of translation triggers Rhodependent transcription termination (2). Thus transcription of rRNA operons present a paradox: how does the transcribing RNA polymerase escape activation of Rho-dependent transcription termination when synthesizing an untranslated transcript over 5,000 nucleotides in length? It has been found in fact that Rho-dependent termination signals present in transposons Tn9 and Tnl0 and the insertion element lSI do not cause termination when these signals are inserted in rRNA operons (22, 118, 152). Also, when rrnB (94) and rrnC (182) promoter-leader regions are transcribed in vitro, premature transcription termination is observed, even in the absence of Rho protein. In addition, when the normal rrnG promoter-leader region and the first 80 nucleotides of the 16S gene are replaced by the araBAD promoter, Rho-dependent transcription termination is observed within the 16S gene (3). In 1984, a model was proposed that accounts for these observations (75, 109). It was hypothesized that RNA polymerase is modified at a site in the rrn leader region, allowing it to read through downstream transcription termination signals. The model is similar to the well-documented anti termination mechanism used during lytic growth of bacteriophage lambda (57). When lambda is switched from lysogeny to lytic growth, two phage anti termination proteins, Nand Q, are formed. Several host proteins (termed Nus factors) and the phage nut sites participate in this antitermination. The E. coli NusA protein is thought to bind transiently to RNA polymerase (69) and to render it susceptible to modification. Apparently, the NusAassociated RNA polymerase recognizes the nut site, where the N protein, and perhaps other Nus factors, interact with the transcription complex and convert it to the anti termination state. Friedman and colleagues (58, 128) have suggested that a nucleotide sequence, termed BoxA and located shortly before the nut site, is required for NusA participation. The Q antitermination system seems to involve a similar but simpler antitermination mechanism. Q anti termination can occur in a purified in vitro system containing only RNA polymerase, an appropriate DNA, and the NusA and Q proteins (67, 68). The nucleotide sequences of six rrn operon leader regions have been reported (rrnD and rrnX [183]; rrnA and rrnE [34]; rrnB [31]; rrnG [151]). The leader sequences are highly conserved, in particular the P2 promoter, a BoxA sequence following it, and the TL region found to cause transcription termination in vitro (Fig. 8A). The role of these sequences in antitermination has been tested by Li et al. (109). A 67-basepair restriction fragment immediately following the rrnG P2 promoter contains the BoxA sequence. When 1295 P2 BoxA rrs in vitro ••- _ _ _ _ _--<. • ._-------in vivo ••- - - - - - - - <. • . _ - - - - - - - - - -_.. _ B P LP,lnaC BoxA InaA ~Z··~····~·~.~--------------~c== • • • +TRP'••-------------------~.~ c P LP livJ ~ • -LEU. .. FIG. 8. Schematic representations of leader regions for genes controlled by unconventional transcription attenuation mechanisms. P, Promoter regions; LP, leader peptide coding regions; T L , a NusA-dependent terminator (94). Lines below the leader region diagrams represent terminated and read through transcripts produced under the indicated conditions. (A) E. coli rrn operon leader regions. (B) E. coli tryptophanase operon (tna) leader region. (e) Leader region of the gene (ilvI) for the E. coli LIV-binding protein. this fragment was placed in front of a known terminator sequence, it caused a 50% decrease in transcription termination (109). Translation through the 67base-pair fragment or reversal of its orientation resulted in a complete loss of antitermination activity. Thus, it is possible that RNA polymerase is modified when it reaches a site early in rRNA operons so that it is resistant to subsequent Rho-dependent and Rhoindependent transcription termination signals. The cellular factors responsible for this modification have not been identified. However, the existence of an anti termination mechanism that participates in rRNA synthesis raises the possibility that there are additional mechanisms for regulation by transcription attenuation. If modification of RNA polymerase could be controlled at specific sites, the cell could modulate the frequency of read through transcription in any operon. Ribosomal Protein Operons Regulation by transcription attenuation has been demonstrated in two ribosomal protein operons. The SlO ribosomal protein operon encodes ribosomal protein components of both the 50S and 30S ribosomal subunits (110, 184). The protein product of the third gene in the operon, protein L4, regulates transcription termination at a site in the leader region of the operon (Ill). The second example of attenuation involves the rplJL-rpoBC operon. This operon contains the structural genes for two 70S ribosomal protein components and the two large subunits of E. coli RNA polymerase. Transcripts that originate upstream of rplK can be terminated at a site between rplL and rpoB (12). 1296 LANDICK AND YANOFSKY In the SlO operon, attenuation shuts off expression of the operon when the level of free L4 protein exceeds that necessary for ribosome assembly. Excess L4 protein also acts as a translational repressor of ribosomal protein synthesis (181), a function also attributed to ribosomal proteins Ll and Ll0l11 (85). The transcript of the SlO operon contains a 172-nucleotide leader region preceding the first gene, rpsJ, encoding SlO (127). Within this region there is an open reading frame potentially coding for a 28-amino acid residue le<>.der peptide. A possible Rho-independent terminator can be formed from a dyad symmetry within the leader peptide open reading frame. Interestingly, the potential RNA hairpin not only is followed by a run of V's consistent with the observed length of an in vivo attenuated transcript (127), but it also contains a nine-base homology to the sequence in the 23S rRNA that can be cross-linked to protein L4 by irradiation with VV light (115). Additional sequence homologies between this region of 23S rRNA and the segment of the SlO transcript past the putative termination site also exist (127). No model has been proposed to describe how protein L4 might interact with these target sequences in the SlO leader region and cause termination at the putative attenuator. Ribosomal protein LlO (rplJ gene product) and the two large subunits of RNA polymerase, f3 and W (products of the rpoB and rpoC genes, respectively), are cotranscribed from one major promoter situated before the rplJ gene (135). Minor promoters also exist preceding rplJ and rpoB (11). The possibility of attenuation between the rplL and rpoB genes was first suspected because ribosomal proteins are present in greater quantities than RNA polymerase subunits (112). Termination occurs in an untranslated segment of RNA that could fold into two successive hairpins followed by four V residues (12). No model explaining attenuation at this site has been proposed. The tna and liv Operons In E. coli and S. typhimurium there are two distinct mechanisms of transcription termination: Rho-dependent and Rho-independent termination (2, 132, 133). Although most of the examples we have described involve Rho-independent termination, it seems reasonable to expect that Rho-dependent attenuation also exists. While anti termination in rRNA operons might be an example of this, the metabolic basis for regulation of these operons has not been established. Recently, two examples of Rho-dependent transcription attenuation have been uncovered. The best characterized of these, tryptophan-mediated induction of expression of the E. coli tryptophanase (Ina) operon, appears to involve an anti termination override of Rho-dependent termination in a leader region (Fig. 8B). The other example, leucine-regulated expression of the gene for the E. coli leucine-, isoleucine-, and valine (LIV)-binding protein that is part of the high-affinity branched-chain amino acid transport system, may involve coupling of the translation of a leader peptide coding region to Rho-dependent transcription termination (Fig. 8e). The tna operon contains at least two genes: tnaA, which encodes tryptophanase, a catabolic enzyme that allows E. coli to grow with tryptophan as the sole source of carbon and energy (35, 124); and tnaB, which codes for a low-affinity tryptophan permease (35, 39). Expression of the tna operon is induced by tryptophan and is subject to catabolic repression. In vitro transcription experiments have located a cyclic AMP-cyclic AMP receptor protein-dependent promoter 319 base pairs upstream from the tnaA initiation codon (36). This 319-nucleotide leader region contains an open reading frame that encodes a 24-amino acid residue leader peptide, designated tnac. The remainder of the leader RNA can base pair to form a number of secondary structures (160), but none resembling the conventional attenuator arrangement has been identified. In vivo, transcription from the tna promoter terminates at a set of discrete sites in the tna leader region (160, 161). Addition of tryptophan to cultures greatly reduces this termination (161). The in vivo termination phenomenon can be closely mimicked in an in vitro transcription system by addition of Rho protein (160). E. coli strains bearing the strong mutant allele rho-IS (161) exhibit semiconstitutive tna operon expression. Furthermore, a lacZ fusion to the early part of the tnaA gene shows normal tryptophan-dependent induction, whereas a lacZ fusion to tnaC is expressed constitutively (161). Taken together, these results suggest that tryptophan induction of tna operon expression is accomplished by suppression of Rhomediated transcription termination in the tna leader region. This hypothesis is supported by the additional finding that all isolated tna constitutive mutations but one have nucleotide changes within a 9-base segment of the tna leader at the end of the tnaC gene. Interestingly, this sequence is homologous to the BoxA sequence thought to be responsible for NusA protein interaction in transcription antitermination (58, 69, 128). It seems likely that transcription attenuation in the tna operon is controlled by an anti termination mechanism that, when triggered by a signal involving tryptophan, overrides Rho-dependent transcription termination in the tna leader region. Antitermination may involve modification of RNA polymerase at the BoxA-like sequence, as has been postulated in the rrn and A tRI systems (57, 75, 109). According to this model, a cellular protein must exist that causes modification only when it is bound to tryptophan. No trans-acting factors have as yet been identified (161; V. Stewart, personal communication). Further understanding of the attenuation mechanism used in tna operon regulation requires elucidation of the basis of tryptophan-media ted anti termina tion. The high-affinity branched-chain amino acid transport system of E. coli is composed of three inner membrane proteins and two periplasmic amino acidbinding proteins with slightly different specificities (123). The system designated LIV-I is induced by leucine limitation and scavenges branched-chain amino acids from the growth medium. The gene for the LIV-binding protein, livJ, is in a transcriptional unit that is separate from the other genes, which apparently form a single operon (123). Early work on the regulation of liv gene expression revealed that mutations in rho and leuS (encoding leucyl-tRNA synthetase) caused elevated levels of LIV-I transport activity (138, 139). Mutations in a previously unde- 77 fined locus, livR, also increased transport (5). The results obtained suggested that expression of the liv genes might be controlled by both repression and attenuation (140). Recent studies have shown that the livi gene and the liv operon contain 124 and 250 nucleotide leader regions, respectively (R. Landick, unpublished results). Both leader regions contain potential short peptide coding regions that contain leucine codons, and long stretches of unstructured, CA-rich sequences that are similar to known Rho-dependent termination sequences. It is possible that translation of the leader peptide is somehow coupled to Rho protein entry on the nascent transcript (99). In such a scenario, stalled translation in response to leucine limitation could block Rho-dependent termination. Clearly this mechanism of regulation is not well defined. Isolation of leader region mutations that result in increased liv gene expression will be required to understand how the postulated Rho-dependent attenuators function. Further analysis of regulation of the tna and liv operons may reveal different ways that Rho-dependent termination is utilized in attenuation. The tna system appears to involve an anti termination function that overcomes Rho-dependent termination, whereas the liv system may couple the efficiency of translation of a leader peptide coding region to Rho-dependent termination. Although both systems require more work, the findings obtained already suggest new variations on the attenuation theme. CONCLUSIONS Transcription attenuation is an attractive regulatory mechanism because it provides regulatory options to the growing cell that are not appropriate to regulation by repression. During the process of transcription, specific RNA sequences and structures are synthesized that present novel opportunities for modulating the course of transcription. These RNA segments may serve as regulatory signals, they may act as sites of interaction with accessory proteins, or, if they contain coding regions, they may allow the cell's translation machinery to influence the course of transcription. In analyses of attenuation in biosynthetic operons we have seen how RNA transcription pause and termination signals are altered by translation to effect a specific regulatory response. One expects that RNA sequences and structures also serve as recognition sites for specific RNA-binding proteins which, when bound, can have regulatory consequences. Some examples of this already exist. In addition, accessory transcription factors such as the NusA, Rho, and Tau proteins, which interact with transcription complexes and modify readthrough or termination, introduce other targets for regulation. Similarly, the subunits of the transcribing polymerase molecule are targets for interaction with or modification by regulatory molecules. Thus the participants and events involved in transcription elongation and transcription termination provide a variety of regulatory opportunities that do not exist in repression. A second feature of attenuation that adds to its attractiveness as a specific regulatory mechanism is its dependence on minimal genetic information. In the biosynthetic operons regulated by attenuation, speci- TRANSCRIPTION ATTENUATION 1297 ficity is imparted by the unique sequence of 100 or so base pairs in the leader region. The normal components of transcription and translation do the rest. By contrast, repressors require a protein coding region and flanking expression-control sequences. In addition, repressor binding must be highly specific so as not to affect expression of genes for unrelated functions. In evolutionary terms, then, relatively few genetic events may be needed to create the leader region of an operon regulated by attenuation. As we have mentioned, extensive sequence and structural homology between tRNA His and the transcript of the his leader region has been noted (4). This homology may result from common ancestry, suggesting a likely source of genetic material for the evolution of regulatory leader regions. A major conclusion that can be drawn from the many regulatory studies performed with E. coli and S. typhimurium is that multiple regulatory mechanisms are commonly used in the control of gene expression. Perhaps this is so because expression of many bacterial genes is influenced by both intracellular and extracellular events and because the functions of these genes are crucial to cell growth. The overall picture that has emerged is that virtually every event involved in gene expression in bacteria has been optimized through the use of some control feature or regulatory mechanism. ACKNOWLEDGMENTS We are greatly indebted to Terry Platt, Irving Crawford, Charles Turnbough, and Valley Stewart for their helpful comments on this review. R.L. was a postdoctoral fellow of the U.S. Public Health Service when this chapter was written, and c.Y. is a Career Investigator of the American Heart Association. LITERATURE CITED I. Adams, C. W., M. Rosenberg, and G. W. Hatfield. 1985. Analysis of in vivo RNA transcription products of the ilvGEDA attenuator region of Escherichia coli K-12. J. Bio!. Chem. 260:8538-8~44. 2. Adhya, S., and M. Gottesman. 1978. Control of transcription termination. Annu. Rev. Biochem. 47:967-996. 3. Aksoy, S., C. L. Squires, and C. Squires. 1984. Evidence for anti termination in Escherichia coli rRNA transcription. J. Bacterio!' 159:260--264. 4. Ames, B. N., T. Tsang, M. Buck. and M. F. Christman. 1983. The leader mRNA of the histidine attenuator region resembles tRNA H ": possible general regulatory implications. Proc. Natl. Acad. Sci. USA 80:5240--5242. 5. Anderson, J. J., S. C. Quay, and D. L. Oxender. 1976. Mapping of two loci affecting the regulation of branched-chain amino acid transport in Escherichia coli. J. Bacteriol. 126:80--90. 6. Artz, S. W., and J. R. Broach. 1975. Histidine regulation in Salmonella typhimurium: an activator-attenuator model of gene regulation. Proc. Nat!. Acad. Sci. USA 72:3453-3457. 7. Artz, S. W., and D. Holzschu. 1983. Histidine biosynthesis and its regulation, p. 379-404. In K. M. Herrmann and R. L. Somerville (ed.), Amino acids: biosyntheSIS and genetic regulation. Addison-Wesley Publishing Co., Reading, Mass. 8. Bachmann, B. J. 1983. Linkage map of Escherichia coli K-12, edition 7. Microbiol. Rev. 47:180--230. 9. Baker. R., and C. Yanofsky. 1972. Transcription initiation frequency and translational yield for the tryptophan operon of Escherichia coli. J. Mo!. Bio!. 69:89-102. 10. Barnes, W. M. 1978. DNA sequence from the histidine operon control region: seven histidine codons in a row. Proc. Natl. Acad. Sci. USA 75:4281-4285. II. Barry, G., C. Squires, and C. L. Squires. 1979. Control features within the rpUL-rpoBC transcription unit of Escherichia coli. Proc. Nat!. Acad. Sci. USA 76:4922-4926. 12. Barry, G., C. Squires, and C. L. Squires. 1980. Attenuation and processing of RNA from rpUL-rpoBC transcription unit of Esch- 1298 LANDICK AND YANOFSKY erichia coli. Proc. Natl. Acad. Sci. USA 77:3331-3335. 13. Bauer, C. E., J. Carey, L. M. Kasper, S. P. Lynn, D. A. Waechter, and J. F. Gardner. 1983. Attenuation in bacterial operons, p. 65-89. In J. Beckwith, J. Davies, and J. Gallant (ed.), Gene functions in prokaryotes. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y. 14. Berlin, V., and C. Yanofsky. 1983. Release of transcript and template during transcription termination at the trp operon attenuator. J. BioI. Chern. 258:1714-1719. 15. Bertrand, K., L.J. Korn, F. Lee, and C. Yanofsky. 1977. The attenuator of the tryptophan operon of Escherichia coli: heterogenous 3'-OH termini in vivo and deletion mapping of functions. J. Mol. BioI. 117:227-247. 16. Bertrand, K., C. Squires, and C. Yanofsky. 1976. Transcription termination in vivo in the leader region of the tryptophan operon of Escherichia coli. J. Mol. BioI. 103:319-337. 17. Bertrand, K., and C. Yanofsky. 1976. Regulation of transcription termination in the leader region of the tryptophan operon of Escherichia coli involves tryptophan or its metabolic product. J. Mol. BioI. 103:339-349. 18. Blasi, F., and C. B. Bruni. 1981. Regulation of the histidine operon: translation-controlled transcription termination (a mechanism common to several biosynthetic pathways). Curr. Top. Cell. Regul. 19:1-45. 19. Bonekamp, F., K. Clemmesen, O. Karlstrom, and K. F. Jensen. 1984. Mechanism of UTP-modulated attenuation at the pyrE gene of Escherichia coli: an example of operon polarity control through the coupling of translation to transcription. EMBO J. 3:2857-2861. 20. Brenchley, J. E., and L. S. Williams. 1975. Transfer RNA involvement in the regulation of enzyme synthesis. Annu. Rev. Microbiol. 29:251-274. 21. Brenner, M., and B. N. Ames. 1971. The histidine operon and its regulation, p. 349-387. In H. S. Vogel (ed.), Metabolic pathways, vol. 5. Academic Press, Inc., New York. 22. Brewster, J. M., and E. A. Morgan. 1981. Tn9 and lSI inserts in a ribosomal ribonucleic acid operon of Escherichia coli are incompletely polar. J. Bacteriol. 148:897-903. 23. Briat, J.-F., and M. J. Chamberlin. 1984. Identification and characterization of a new transcriptional termination factor from Escherichia coli. Proc. Natl. Acad. Sci. USA 81:7373-7377. 24. Calvo, J. M., M. Freundlich, and H. E. Umbarger. 1969. Regulation of branched-chain amino acid biosynthesis in Salmonella typhimurium: isolation of regulatory mutants. J. Bacteriol. 97:1272-1282. 25. Calvo, J. M., P. Margolin, and H. E. Umbarger. 1969. Operator constitutive mutations in the leucine operon of Salmonella typhimurium. Genetics 61:777-787. 26. Carter, P. W., D. L. Weiss, H. L. Weith, and J. M. Calvo. 1985. Mutations that convert the four leucine codons of the Salmonella typhimurium leu leader to four threonine codons. J. Bacteriol. 162:943-949. 27. Clemmesen, K., F. Bonekamp, O. Karlstrom, and K. J. Jensen. 1985. Role of translation in the UTP-modulated attenuation at the pyrBI operon of Escherichia coli. Mol. Gen. Genet. 201:247-251. 28. Cohen, G., and F. Jacob. 1959. Sur la repression de la synthese des enzymes intervenent dans la formations du tryptophane chez Escherichia coli. C. R. Acad. Sci. 248:3490--3492. 29. Comer, M. M. 1982. Threonine tRNAs and their genes in Escherichia coli. Mol. Gen. Genet. 187:132-137. 30. Comer, M. M., and A. Bock. 1976. Genes for the 0: and 13 subunits of the phenylalanine transfer ribonucleic acid synthetase of Escherichia coli. J. Bacterial. 127:923-933. 31. Csordas-Toth, E., I. Boros, and P. Venetianer. 1979. Structure of the promoter region of the rrnB gene in Escherichia coli. Nucleic Acids Res. 7:2189-2197. 32. Das, A., I. P. Crawford, and C. Yanofsky. 1982. Regulation of tryptophan operon expression by attenuation in cell-free extracts of Escherichia coli. J. BioI. Chern. 257:8795-8798. 33. Das, A., J. Urbanowski, H. Weissbach, J. Nestor, and C. Yanofsky. 1983. In vitro synthesis of the tryptophan operon leader peptides of Escherichia coli, Serratia marcescens and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 80:28792883. 34. deBoer, H. A., S. F. Gilbert, and M. Nomura. 1979. DNA sequences of promoter regions for rRNA operons rrnE and rrnA in E. coli. Cell 17:201-209. 35. Deeley, M.C., and C. Yanofsky. 1981. Nucleotide sequence of the structural gene for tryptophanase of Escherichia coli K-12. J. Bacteriol. 147:787-796. 36. Deeley, M. C., and C. Yanofsky. 1982. Transcription initiation at the tryptophanase promoter of Escherichia coli K-12. J. Bacteriol. 151:942-951. 37. Dekel-Gorodetsky, L., R. Schoulaker-Schwarz, and H. Engelberg-Kulka. 1986. Escherichia coli tryptophan operon directs the in vivo synthesis of a leader peptide. J. Bacteriol. 165:1046-1048. 38. DiNocera, P. P., F. Blasi, R. DiLauro, R. Frunzio, and C. B. BrunI. 1978. Nucleotide sequence of the attenuator region of the histidine operator of Escherichia coli K-12. Proc. Natl. Acad. Sci. USA 75:4276-4280. 39. Edwards, R. M., and M. D. Yudkin. 1984. Tryptophanase synthesis in Escherichia coli: the role of indole replacement in supplying tryptophan and the nature of the constitutive mutation tnaR3. J. Gen. Microbiol. 130:1535-1542. 40. Eidlic, L., and F. C. Neidhardt. 1965. Role of valyl-tRNA synthetase in enzyme repression. Proc. Natl. Acad. Sci. USA 53:539-543. 41. Eisenberg, S. P., L. Soli, and M. Yarus. 1980. Role of tRNAT", and leader RNA secondary structure in attenuation of the trp operon, p. 469-479. In D. Soli, P. Schimmel, and J. Abelson (ed.), Transfer RNA: biological aspects. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y. 42. Eisenberg, S. P., M. Yarus, and L. Soli. 1979. The effect of an Escherichia coli regulatory mutation on transfer RNA structure. J. Mol. BioI. 135:111-126. 43. Ellwood, M., and M. Nomura. 1982. Chromosomal locations of the genes for rRNA in Escherichia coli K-12. J. Bacteriol. 149:459-468. 44. Farnham, P. J., J. Greenblatt, and T. Platt. 1982. Effects of nusA protein on transcription termination of the tryptophan operon of E. coli. Cell 29:945-951. 45. Farnham, P.J., and T. Platt. 1980. A model for transcription termination suggested by studies on the trp attenuator in vitro using base analogs. Cell 20:739-748. 46. Farnham, P. J., and T. platt. 1981. Rho-independent termination: dyad symmetry in DNA causes RNA polymerase to pause during transcription in vitro. Nucleic Acids Res. 9:563-577. 47. Farnham, P. J., and T. platt. 1982. Effects of DNA base analogs on transcription termination at the tryptophan operon attenuator in Escherichia coli. Proc. Natl. Acad. Sci. USA 79:998-1002. 48. Fayat, G. M., J.-F. Mayaux, C. Sacerdot, M. Fromant, M. Springer, M. Grunberg-Manago, and S. Blanquet. 1983. Escherichia coli phenylalanyl-tRNA synthetase operon. Evidence for an attenuation mechanism. Identification of the gene for the ribosomal protein L20. J. Mol. BioI. 171:239-261. 49. Fisher, R. F., A. Das, R. Kolter, M. E. Winkler, and C. Yanofsky. 1985. Analysis of the requirements for transcription pausing in the tryptophan operons. J. Mol. BioI. 182:397-409. 50. Fisher, R. F., and C. Yanofsky. 1983. A complementary DNA oligomer releases a transcription pause complex. J. BioI. Chern. 258:9208-9212. 51. Fisher, R. F., and C. Yanofsky. 1983. Mutations of the beta subunit of RNA alter both transcription pausing and transcription termination in the trp operon leader region. J. BioI. Chern. 258:8146-8150. 52. Fisher, R. F., and C. Yanofsky. 1984. Use of complementary DNA oligomers to probe trp leader transcript secondary structures involved in transcription pausing and termination. Nucleic Acids Res. 12:3295-3302. 53. Freedman, R., and P. Schimmel. 1981. 111 vitro transcription of the histidine operon. J. BioI. Chern. 256:10747-10750. 54. Freundlich, M. 1963. Multivalent repression in the biosynthesis of threonine in Salmonella typhimurium and Escherichia coli. Biochem. Biophys. Res. Commun. 10:277-282. 55. Freundlich, M. 1977. Cyclic AMP can replace the relA-dependent requirement for derepression of acetohydroxy acid synthetase in E. coli K-12. Cell 12:1121-1126. 56. Friden, P., T. Newman, and M. Freundlich. 1982. Nucleotide sequence of the ilvB promoter-regulatory region: a biosynthetic operon controlled by attenuation and cyclic AMP. Proc. Natl. Acad. Sci. USA 79:6156-6160. 57. Friedman, D.l., and M. Gottesman. 1983. Lytic mode of lambda development, p. 21-51. In R. W. Hendrix, 1. W. Roberts, F. W. Stahl, and R. A. Weisberg (ed.), Lambda II. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y. 58. Friedman, D. 1., and E. R. Olson. 1983. Evidence that a nucleotide sequence, "boxA," is involved in the action of the NusA protein. Cell 34:143-149. 59. Friedman, D.l., E. R. Olson, C. Georgopoulos, K. Tilly, I. Hershkowitz, and F. Banuett. 1984. Interactions of bacteriophage and host macromolecules in the growth of bacteriophage A. Microbiol. Rev. 48:299-325. 77 60. Fruzio, R., C. B. Bruni, and F. Blasi. 1981. In vivo and in vitro detection of the leader RNA of the histidine operon of Escherichia coli K-12. Proc. Natl. Acad. Sci. USA 78:2767-2771. 61. Gardner, J. F. 1979. Regulation of the threonine operon: tandem threonine and isoleucine codons in the control region and translational control of transcription termination. Proc. Natl. Acad. Sci. USA 76:1706-1710. 62. Gardner, J. F. 1982. Initiation, pausing and termination of transcription in the threonine operon regulatory region of Escherichia coli. J. BioI. Chern. 257:3896-3904. 63. Gardner, J. F., and W. S. ReznikolI. 1978. Identification and restriction endonuclease mapping of the threonine operon regulatory region. J. Mol. BioI. 126:241-258. 64. Gemmill, R. M., S. R. Wessler, E. B. Keller, and J. M. Calvo. 1979. leu operon of Salmonella typhimurium is controlled by an attenuation mechanism. Proc. Natl. Acad. Sci. USA 76:49414945. 65. Gowishankar, J., and J. Pittard. 1982. Regulation of phenylalanine biosynthesis in Escherichia coli K-12: control of transcription of the pheA operon. J. Bacteriol. 150:1130-1137. 66. Grantham, R. C., M. Gautier, M. Gouy, M. Jacobzone, and R. Mercier. 1981. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 9:r43-r74. 67. Grayhack, E. J., andJ. W. Roberts. 1982. The phage A Q product: activity of a transcription anti terminator in vitro. Cell 30:637-648. 68. Grayhack, E. J., X. Yang, L. F. Lau, and J. W. Roberts. 1985. Phage A gene Q product antiterminator recognizes RNA polymerase near the promoter and accelerates it through a pause site. Cell 42:259-269. 69. Greenblatt, J., and J. Li. 1981. Interaction of sigma factor and the nusA gene protein of E. coli with RNA polymerase in the initiation-termination cycle of transcription. Cell 24:421-428. 70. Harms, E., J.-H. Hsu, C. S. Subrahmanyam, and H.E. Umbarger. 1985. Comparison of the regulatory regions of the ilvGEDA operons from several enteric organisms. J. Bacteriol. 164: 207-216. 71. Hauser, C. A., and G. W. Hatfield. 1983. Nucleotide sequence of the ilvB multivalent attenuator region of Escherichia coli K12. Nucleic Acids Res. 1l:127-131. 72. Hauser, C. A., and G. W. Hatfield. 1984. Attenuation of the ilvB operon by amino acids reflecting substrates or products of the ilvB product. Proc. Natl. Acad. Sci. USA 81:76-79. 73. Hauser, C. A., J. A. Sharp, L. K. Hatfield, and G W. Hatfield. 1985. Pausing of RNA polymerase during in vitro transcription through the ilvB and ilvGEDA attenuator regions of Escherichia coli K-12. J. BioI. Chern. 260:1765-1770. 74. Hlraga, S., and C. Yanofsky. 1973. Inhibition of the progress of transcription on the tryptophan operon of Escherichia coli. J. Mol. BioI. 79:339-349. 75. Holden, W. E., and E. A. Morgan. 1984. Antitermination of transcription from an Escherichia coli ribosomal RNA promoter. Proc. Natl. Acad. Sci. USA 81:6789-6793. 76. Hsu, J.-H., E. Harms, and H. E. Umbarger. 1985. Leucine regulation of the ilvGEDA operon of Serratia marcescens by attenuation is modulated by a single leucine codon. J. Bacteriol. 164:217-222. 76a.Hudson, G. S., and B. E. Davidson. 1984. Nucleotide sequence and transcription of the phenylalanine and tyrosine operons of Escherichia coli K-12. J. Mol. BioI. 180:1023-1051. 77. Imamoto, F. 1968. Immediate cessation of transcription of the operator-proximal region of the tryptophan operon of E. coli after repression of the operon. Nature (London) 220:31-34. 78. Ingraham, J. L., O. Maaloe, and F. C. Neidhardt. 1983. Growth of the bacterial cell, p. 349-385. Sinauer Assoc., Sunderland, Mass. 79. Jackson, E. N., and C. Yanofsky. 1972. Internal promoter of the tryptophan operon of Escherichia coli is located in a structural gene. J. Mol. BioI. 69:307-313. 80. Jackson, E. N., and C. Yanofsky. 1973. The region between the operator and first structural gene of the tryptophan of Escherichia coli may have a regulatory function. J. Mol. BioI. 76:89-101. 81. Jacob, F., and J. Monod. 1961. Genetic regulatory mechanisms in the synthesis of proteins. J. Mol. BioI. 3:318-356. 82. Jaurin, B., T. Grundstrom, T. Edlund, and S. J. Normark. 1981. The E. coli J3-lactamase attenuator mediates growth rate regulation. Nature (London) 290:221-225. 83. Jaurin, B., and S.J. Normark. 1979. In vivo regulation of chromosomal J3-lactamase in Escherichia coli. J. Bacteriol. 138:896-902. 84. Jensen, K. F., J. Neuhard, and L. Schack. 1982. RNA polymerase 85. 86. 87. 88. 89. 90. 91. 92. 93. 94. 95. 96. 97. 98. 99. 100. 101. 102. 103. 104. 105. 106. 107. TRANSCRIPTION ATTENUATION 1299 involvement in the regulation of expression of Salmonella typhimurium pyr genes. Isolation and characterization of a fluorouracil-resistant mutant with high constitutive expression of the pyrB and pyrE genes due to a mutation in rpoBe. EMBO J. 1:69-74. Johnsen M., T. Christensen, P. P. Dennis, and N. P. Fill. 1982. Autogenous control: ribosomal protein LlO-Ll2 complex binds to the leader sequence of its mRNA. EMBO J. 1:999-1004. Johnston, H. M., W. M. Barnes, F. G. Chumley, L. Bossi, and J. R. Roth. 1980. Model for regulation of the histidine operon of Salmonella. Proc. Natl. Acad. Sci. USA 77:508-512. Johnston, H. M., and J. R. Roth. 1979. Histidine mutants requiring adenine: selection of mutants with reduced hisG expression in Salmonella typhimurium. Genetics 92:1-15. Johnston, H. M., and J. R. Roth. 1981. Genetic analysis of the histidine operon control region of Salmonella typhimurium. J. Mol. BioI. 145:731-734. Johnston, H. M., and J. R. Roth. 1981. DNA sequence changes of mutations altering attenuation control of the histidine operon control region of Salmonella typhimurium. J. Mol. BioI. 145: 735-756. Kasai, T. 1974. Regulation of the expression of the histidine operon in Salmonella typhimurium. Nature (London) 249:523527. Kassavetis, G. A., and M. J. Chamberlin. 1981. Pausing and termination of transcription within the early region of bacteriophage T7 DNA in vitro. J. BioI. Chern. 256:2777-2786. Keller, E. B., and J. M. Calvo. 1979. Alternative secondary structures of leader RNAs and the regulation of the trp, phe, hi;, thr and leu operons. Proc. Nat!. Acad. Sci. USA 76:6186-6190. Kelln, R. A., J. J. Kinahan, K. F. Foltermann, and G. A. O'Donovan. 1975. Pyrimidine biosynthetic enzymes of Salmonella typhimurium, repressed specifically by growth in the presence of cytidine. J. Bacteriol. 124:764-774. Kingston, R. E., and M. J. Chamberlin. 1981. Pausing and attenuation of in vitro transcription in the rrnB operon of E. coli. Cel! 27:523-531. Kltchlngman, G. R., and M. J. Fournier. 1977. Modificationdeficient transfer ribonucleic acids from relaxed control Escherichia coli: structures of the major undermodified phenylalanine and leucine transfer RNAs produced during leucine starvation. Biochemistry 16:2213-2220. Kolter, R., and C. Yanofsky. 1982. Attenuation in amino acid biosynthetic operons. Annu. Rev. Genet. 16:113-143. Kolter, R., and C. Yanofsky. 1984. Genetic analysis of the tryptophan operon regulatory region using site-directed mutagenesis. J. Mol. BioI. 175:299-312. Kuroda, M. I., and C. Yanofsky. 1984. Evidence for the transcript secondary structures predicted to regulate transcription attenuation in the trp operon. J. BioI. Chern. 259:12838-12843. Landlck, R. 1984. Regulation of LIV-I transport system gene expression, p. 71-74. In L. Leive and D. Schlessinger (cd.), Microbiology-1984. American Society for Microbiology, Washington, D.C. Landick, R., J. Carey, and C. Yanofsky. 1985. Translation activates the paused transcription complex and restores transcription of the trp operon leader region. Proc. Natl. Acad. Sci. USA 82:4663-4667. Landlck, R., and C. Yanofsky. 1984. Stability of an RNA secondary structure affects in vitro transcription pausing in the trp operon leader region. 1. BioI. Chern. 259:11550-11555. Lawther, R. P., D. H. Calhoun, C. W. Adams, C. A. Hauser, J. Gray, and G. W. Hatfield. 1981. Molecular basis of valine resistance in Escherichia coli K-12. Proc. Natl. Acad. Sci. USA 78:922-925. Lawther, R. P., and G. W. Hatfield. 1980. Multivalent translational control of transcription termination at the attenuator of ilvGEDA of Escherichia coli. Proc. Natl. Acad. Sci. USA 77:1862-1866. Lee, F., K. Bertrand, G. Bennett, and C. Yanofsky. 1978. Comparison of the nucleotide sequences of the initial transcribed regions of the tryptophan operons of Escherichia coli and Salmonella typhimurium. J. Mol. BioI. 121:193-217. Lee, F., C. L. Squires, C. Squires, and C. Yanofsky. 1976. Termination of transcription in vitro in the Escherichia coli tryptophan operon leader region. J. Mol. BioI. 103:383-393. Lee, F., and C. Yanofsky. 1977. Transcription termination at the trp operon attenuators of Escherichia coli and Salmonella typhimurium: RNA secondary structure and regulation of termination. Proc. Natl. Acad. Sci. USA 74:4365-4369. Levin, H., and H. K. Schachman. 1985. Regulation of aspartate transcarbamoylase synthesis in Escherichia coli: analysis of 1300 108. 109. 110. 111. 112. 113. 114. 115. 116. 117. 118. 119. 120. 121. 122. 123 124. 125. 126. 127. 128. 129. 130. LANDICK AND YANOFSKY deletion mutations in the promoter region of the pyrBI operon. Proc. Natl. Acad. Sci. USA 82:4643-4647. Lewis, J. A., and B. N. Ames. 1972. Histidine regulation in Salmonella typhimurium: the percentage of transfer RNA H ', charged in vivo and its relation to the repression of the histidine operon. J. Mol. BioI. 66:131-142. Li, S. C., C. L. Squires, and C. Squires. 1984. Antitermination of E. coli rRNA transcription is caused by a control region segment containing lambda nut-like sequences. Cell 38:851-860. Lindahl, L., R. H. Archer, and J.-M. Zengel. 1982. Regulation of an eleven gene ribosomal protein operon, p. 105-117. In M. Grunberg-Manago and B. Safer (ed.), Interaction of translational and transcriptional controls in the regulation of gene expression. Elsevier Biomedical Press, New York. Lindahl, L., R. Archer, and J.-M. Zengel. 1983. Transcription of the S 10 ribosomal protein operon is regulated by an attenuator in the leader. Cell 33:241-248. Lindahl, L., M. Yamamoto, M. Nomura, J. B. Kirschbaum, B. Allet, and J.-D. Rochaix. 1977. Mapping of a cluster of genes for components of the transcriptional and translational machineries of Escherichia coli. J. Mol. BioI. 109:23-47. Lynn, S. P., C. E. Bauer, K. Chapman, and J. F. Gardner. 1985. Identification and characterization of mutants affecting transcription termination at the threonine operon attenuator. J. Mol. BioI. 183:529-541. Lynn, S. P., J. F. Gardner, and W. S. Reznikoff. 1982. Attenuation regulation in the thr operon of Escherichia coli K-12: molecular cloning and transcription of the controlling region. J. Bacteriol. 152:363-371. Maly, P., J. Rinke, E. Ulmer, C. Zweib, and R. Brimacombe. 1980. Precise localization of the site of cross-linking between protein L4 and 23S ribonucleic acid induced by mild ultraviolet irradiation of Escherichia coli 50S ribosomal subunits. Biochemistry 19:4179-4188. Mechulam, Y., G. Fayat, and S. Blanquet. 1985. Sequence of the Escherichia coli pheST operon and identification of the himA gene. J. Bacteriol. 163:787-791. Miozzari, G., and C. Yanofsky. 1978. Translation of the leader region of the Escherichia coli tryptophan operon. J. Bacteriol. 133:1457-1466. Morgan, E. A. 1980. Insertions of Tn 10 into an E. coli ribosomal RNA operon are incompletely polar. Cell 21:257-265. Morse, D. E., and A. N. C. Morse. 1976. Dual-control of the tryptophan operon is mediated by both tryptophanyl-tRNA synthetase and the repressor. J. Mol. BioI. 103:209-226. Mosteller, R. D., and C. Yanofsky. 1971. Evidence that tryptophanyl transfer ribonucleic acid is not the corepressor of the tryptophan operon of Escherichia coli. J. Bacteriol. 105:268275. Nargang, F. E., C. S. Subrahmanyam, and H. E. Umbarger. 1980. Nucleotide sequence of ilvGEDA operon attenuator region of Escherichia coli. Proc. Natl. Acad. Sci. USA 77:1823-1827. Navre, M., and H. K. Schachman. 1983. Synthesis of asparate transcarbamoylase in Escherichia coli: transcription regulation of the pyrB-pyrI operon. Proc. Natl. Acad. Sci. USA 80:12071211. Nazos, P. M., T. Z. Su, R. Landick, and D. L. Oxender. 1984. Branched-chain amino acid transport in Escherichia coli, p. 24-28. In L. Leive and D. Schlessinger (cd.), Microbiology1984. American Society for Microbiology, Washington, D.C. Ng, H., and T. H. Gartner. 1963. Selection of mutants of Escherichia coli constitutive for tryptophanase. J. Bacteriol. 85: 245-246. Normark, S., and L. G. Burman. 1977. Resistance of Escherichia coli to penicillins: fine structure mapping and dominance of chromosomal beta-lactamase mutations. J. Bacteriol. 132:1-7. O'Donovan, G. A., and J. Neuhard. 1970. Pyrimidine metabolism in microorganisms. Bacteriol. Rev. 34:278-343. Olins, P.O., and M. Nomura. 1981. Regulation of the S 10 ribosomal proteins of operon i.n E. coli: nucleotide sequences at the start of the operon. Cell 26:205-211. Olson, E. R., E. L. Flamm, and D. I. Friedman. 1982. Analysis of nutR: a region of phage lambda required for antitermination of transcription. Cell 31:61_70. Oxender, D. L., G. Zurawski, and C. Yanofsky. 1979. Attenuation in the Escherichia coli tryptophan operon: role of RNA secondary structure involving the tryptophan codon region. Proc. Natl. Acad. Sci. USA 76:5524-5528. Parsot, C., I. Saint-GIrons, and P. Cossart. 1982. DNA sequence changes of a deletion mutation abolishing attenuation control of the threonine operon of E. coli K12. Mol. Gen. Genet. 188:455458. 131. Pierard, A., N. Glansdorff, D. GIgot, M. Crabeel, D. Halleux, and L. Thiry. 1976. Repression of Escherichia coli carbomoylphosphate synthase: relationships with enzyme synthesis in the arginine and pyrimidine pathways. J. Bacteriol. 127:291-301. 132. Platt, T. 1986. Transcription termination and the regulation of gene expression. Annu. Rev. Biochem. 55:339-372. 133. Platt, T., and D. G. Bear. 1983. Role of RNA polymerase, p factor, and ribosomes in transcription termination, p. 123-161. In J. Beckwith, J. Davies, and J. Gallant (ed.), Gene functions in prokaryotes. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y. 134. Platt, T., C. Squires, and C. Yanofsky. 1976. Ribosome-protected regions of the leader-trpE sequence of Escherichia coli tryptophan operon messenger RNA. J. Mol. BioI. 103:411-420. 135. Post, L. E., G. D. Strycharz, M. Nomura, H. Lewis, and P. P. Dennis. 1979. Nucleotide sequence for the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit ~ in Escherichia coli. Proc. Natl. Acad. Sci. USA 76: 1697-1701. 136. Poulsen, P., F. Bonekamp, and K. F. Jensen. 1984. Structure of the Escherichia coli pyrE operon and control of pyrE expression by a UTP modulated intercistronic attenuation. EMBO J. 3: 1783-1790. 137. Putney, S., and P. Schimmel. 1981. An amino acyl tRNA synthetase binds to a specific DNA sequence and regulates its gene transcription. Nature (London) 291:632-635. 138. Quay, S. C., and D. L. Oxender. 1976. Regulation of branchedchain amino acid transport in Escherichia coli. 1. Bacterial. 127:1225-1238. 139. Quay, S. C., and D. L. Oxender. 1977. Regulation of amino acid transport in Escherichia coli by transcription termination factor rho. J. Bacteriol. 130:1024-1029. 140. Quay, S. C., and D. L. Oxender. 1980. Regulation of membrane transport, p. 413-436. In R. Goldberg (ed.), Biological regulation and development, vol. 2. Plenum Publishing Corp., New York. 141. Roghanl, A., R. C. Gumport, and J. F. Gardner. 1985. Sitedirected mutagenesis in the study of the molecular mechanism of attenuation in the threonine operon of Escherichia coli. UCLA Symp. Mol. Cell. BioI. 30:553-564. 142. Roland, K. L., F. E. Powell, and C. E. Turnbough. 1985. Role of translation and attenuation in the control of pyrBI operon expression in Escherichia coli K-12. 1. Bacteriol. 163:991-999. 143. Roof, W. D., K. F. FeItermann, and J. R. Wild. 1982. The organization and regulation of the pyrBI operon in E. coli includes a p-independent attenuator sequence. Mol. Gen. Genet. 187:391400. 144. Rosenberg, M., and D. Court. 1979. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu. Rev. Genet. 13:319-353. 145. Roth, J. R., D. F. Silbert, G. R. Fink, M. J. Voll, D. Anton, P. E. Hartman, and B. N. Ames. 1966. Transfer RNA and control of the histidine operon. Cold Spring Harbor Symp. Quant. BioI. 31:383-392. 146. Ryan, T., and M. J. Chamberlin. 1983. Transcription analyses with heteroduplex trp attenuator templates indicate that the transcript stem and loop structure serves as the termination signal. J. BioI. Chern. 258:4690-4693. 147. Saint-Girons, I. 1978. New regulatory mutations affecting the expression of the threonine operon in E. coli K-12. Mol. Gen. Genet. 162:95-100. 148. SchleSinger, S., and B. Magasanik. 1964. Effect of a-methylhistidine on the control of histidine synthesis. J. Mol. BioI. 9: 670-682. 149. Schwartz, M., and J. Neuhard. 1975. Control of expression oftbe genes in Salmonella typhimurium: effects of variations in uridine and cytidine nucleotide pools. J. Bacteriol. 121:814-822. 150. Searles, L. L., S. R. Wessler, and J. M. Calvo. 1983. Transcription attenuation is the major mechanism by which the leu operon of Salmonella typhimurium is controlled. 1. Mol. BioI. 162:377-394. 151. Shen, W.-F., C. Squires, and C. L. Squires. 1982. Nucleotide sequence of the rrnG ribosomal RNA promoter region of Escherichia coli. Nucleic Acids Res. 10:3303-3313. 152. Siehnel. R., and E. A. Morgan. 1983. Efficient readthrough of Tn9 and IS} by RNA polymerase molecules that initiate at rRNA promoters. J. Bacteriol. 153:672-684. 153. Singer, C., G. Smith, R. Cortese, and B. Ames. 1972. Mutant tRNAH ', ineffective in repression and lacking two pseudouridine modifications. Nature (London) 238:72-74. 154. Smith, J. M., F. J. Smith, and H. E. Umbarger. 1979. Mutations affecting the formation of acetohydroxy acid syntase II in Escherichia coli K-12. Mol. Gen. Genet. 169:299-314. 77 155. Sommer, H., A. Schmitz, U. Schmeissner, J. H. Miller, and H. Wittman. 1978. Genetic studies of the lac repressor. VI. The B116 repressor. J. Mol. BioI. 123:457-469. 156. Springer, M., J.-F. Mayaux, G. Fayat, M. Graffe, S. Blanquet, and M. Grunberg-Manago. 1985. Attenuation control of the Escherichia coli phenylalanyl-tRNA synthetase operon. J. Mol. BioI. 181:467-478. 157. Springer, M., J. A. Plumbridge, J. S. Butler, M. Graffe, J. Dondon, J.-F. Mayaux, G. Fayat, P. Lestienne, S. Blanquet, and M. Grunberg-Manago. 1985. Autogenous control of Escherichia coli threonyl-tRNA synthetase expression in vivo. J. Mol. BioI. 185:93-104. 158. Springer, M., M. Trudel, M. Graffe, J. A. Plumbridge, G. Fayat, J.-F. Mayaux, C. Sacerdot, S. Blanquet, and M. GrunbergManago. 1983. Escherichia coli phenylalanyl-tRNA synthetase operon is controlled by attenuation in vivo. J. Mol. BioI. 171:263-279. 159. Stauffer, G. V., G. Zurawski, and C. Yanofsky. 1978. Single base-pair alterations in the Escherichia coli trp operon leader region that relieve transcription termination at the trp attenuator. Proc. Natl. Acad. Sci. USA 75:4833-4837. 160. Stewart, V., R. Landick, and C. Yanofsky. 1986. Rho-dependent transcription termination in the tryptophanase operon leader region of Escherichia coli K-12. J. Bacteriol. 166:217-233. 161. Stewart, V., and C. Yanofsky. 1985. Evidence for transcription anti termination control of tryptophanase gene expression in Escherichia coli K-12. J. Bacteriol. 164:731-740. 162. Stroynowski, I., M. Kuroda, and C. Yanofsky. 1983. Transcription termination in vitro at the tryptophan operon attenuator is controlled by secondary structures in the leader transcript. Proc. Natl. Acad. Sci. USA 80:2206-2210. 163. Stroynowski, I., M. van Cleemput, and C. Yanofsky. 1982. Superattenuation in the tryptophan operon of Serratia marcescens. Nature (London) 298:38-41. 164. Stroynowski, I., and C. Yanofsky. 1982. Transcript secondary structures regulate transcription termination at the attenuator in Serratia marcescens. Nature (London) 298:34-38. 165. Sutton, A., and M. Freundlich. 1980. Regulation by cyclic AMP of the ilvB-encoded biosynthetic acetohydroxy acid synthetase in Escherichia coli K-12. Mol. Gen. Genet. 178:179-183. 166. Theze, J., and I. Saint-Girons. 1974. Threonine locus of Escherichia coli K-12: genetic structure and evidence for an operon. J. Bacteriol. 118:990--998. 167. Tsui, P., and M. Freundlich. 1985. Starvation for ilvB operon leader amino acids other than leucine or valine does not increase acetohydroxy acid synthase activity in Escherichia coli. J. Bacteriol. 162:1314-1316. 168. Turnbough, C. L., K. L. Hicks, and J. P. Donahue. 1983. Attenuation control of pyrBI expression in Escherichia coli K-12. Proc. Natl. Acad. Sci. USA 80:368-372. 169. Turnbough, C. L., R. Neill, R. Landsberg, and B. N. Ames. 1979. Pseudouridylation of tRNAs and its role in regulation in Salmonella typhimurium. J. BioI. Chern. 254:5111-5119. 170. Void, B. S., J. M. Lazar, and A. M. Gray. 1979. Characterization of a deficiency of N 6-(t1 2 -isopentenyl)-2-methylthioadenosine in the Escherichia coli mutant trpX by use of antibodies to N6_(t1 2 _ isopentenyl)adenosine. J. BioI. Chern. 254:7362-7367. 171. Wek, R. C., C. A. Hauser, and G. W. Hatfield. 1985. The nucleotide sequence of the ilvBN operon of Escherichia coli: sequence 172. 173. 174. 175. 176. 177. 178. 179. 180. 181. 182. 183. 184. 185. 186. 187. 188. TRANSCRIPTION ATTENUATION 1301 homologies of the acetohydroxy acid synthase isozymes. Nucleic Acids Res. 13:3995-4010. Wessler, S. R., and J. M. Calvo. 1981. Control of leu operon expression in Escherichia coli by a transcription attenuation mechanism. J. Mol. BioI. 149:579-597. Winkler, M. E., K. Mullis, J. Barnett, I. Stroynowski, and C. Yanofsky. 1982. Transcription termination at the tryptophan operon attenuator is decreased in vitro by an oligomer complementary to a segment of the leader transcript. Proc. Natl. Acad. Sci. USA 79:2181-2185. Winkler, M. E., and C. Yanofsky. 1981. Pausing of RNA polymerase during in vitro transcription of the tryptophan operon leader region. Biochemistry 20:3738-3744. Yanofsky, C. 1981. Attenuation in the control of expression of bacterial operons. Nature (London) 289:751-758. Yanofsky, C. 1982. Attenuation in the control of tryptophan operon expression, p. 17-24. In M. Grunberg-Manago and B. Safer (ed.), Interaction of translational and transcriptional controls in the regulation of gene expression. Elsevier Biomedical Press, New York. Yanofsky, C. 1984. Comparison of regulatory and structural regions of genes of tryptophan metabolism. Mol. BioI. Evol. 1:143-161. Yanofsky, C., and V. Horn. 1981. Rifampin resistance mutations that alter the efficiency of transcription termination at the tryptophan operon attenuator. J. Bacteriol. 145:1334-1341. Yanofsky, C., R. L. Kelley, and V. Horn. 1984. Repression is relieved before attenuation in the trp operon of Escherichia coli as tryptophan starvation becomes increasingly severe. J. Bacteriol. 158:1018-1024. Yanofsky, C., and L. Soli. 1977. Mutations affecting tRNATcp and its charging and their effect on regulation of transcription termination at the attenuator of the trytophan operon. J. Mol. BioI. 113:663-677. Yates, J.-L., and M. Nomura. 1980. E. coli ribosomal protein L4 is a feedback regulatory protein. Cell 21:517-522. Young, R. A. 1979. Transcription termination in the Escherichia coli ribosomal RNA operon rrnC. J. BioI. Chern. 254:1272512731. Young, R. A., and J. A. Steitz. 1979. Tandem promoters direct E. coli ribosomal RNA synthesis. Cell 17:225-234. Zengel, J.-M., D. MueckI. and L. Lindahl. 1980. Protein L4 of the E. coli ribosome regulates an eleven gene r-protein operon. Cell 21 :523-525. Zuker, M., and P. Steigler. 1981. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 9:133-148. Zurawski, G., K. Brown, D. Killingly, and C. Yanofsky. 1978. Nucleotide sequence of the leader region of the phenylalanine operon of Escherichia coli. Proc. Natl. Acad. Sci. USA 75:4271-4275. Zurawski, G., D. Elseviers, G. Stauffer, and C. Yanofsky. 1978. Translational control of transcription termination at the attenuator of the E. coli tryptophan operon. Proc. Natl. Acad. Sci. USA 75:5988-5992. Zurawski, G., and C. Yanofsky. 1980. Escherichia coli tryptophan operon leader mutations, which relieve transcription termination, are cis-dominant to trp leader mutations which increase transcription termination. J. Mol. BioI. 142:123-129.