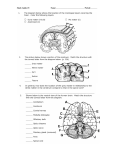
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 123COM.CHP:Corel VENTURA
Human multitasking wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Emotional lateralization wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neurogenomics wikipedia , lookup
Neuroscience and intelligence wikipedia , lookup
Neuroinformatics wikipedia , lookup
Affective neuroscience wikipedia , lookup
Synaptic gating wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Neural modeling fields wikipedia , lookup
Neural oscillation wikipedia , lookup
Brain–computer interface wikipedia , lookup
Neuromarketing wikipedia , lookup
Blood–brain barrier wikipedia , lookup
Binding problem wikipedia , lookup
Environmental enrichment wikipedia , lookup
Brain morphometry wikipedia , lookup
Optogenetics wikipedia , lookup
Nervous system network models wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neurolinguistics wikipedia , lookup
Neurophilosophy wikipedia , lookup
Brain Rules wikipedia , lookup
Selfish brain theory wikipedia , lookup
Time perception wikipedia , lookup
Evoked potential wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Magnetoencephalography wikipedia , lookup
Neurotechnology wikipedia , lookup
Intracranial pressure wikipedia , lookup
Neuropsychology wikipedia , lookup
Cortical cooling wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Sports-related traumatic brain injury wikipedia , lookup
Development of the nervous system wikipedia , lookup
Aging brain wikipedia , lookup
Neural engineering wikipedia , lookup
Neuroplasticity wikipedia , lookup
Human brain wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Neuroesthetics wikipedia , lookup
Neuroeconomics wikipedia , lookup
Functional magnetic resonance imaging wikipedia , lookup
Cerebral cortex wikipedia , lookup
Neural binding wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
History of neuroimaging wikipedia , lookup
CC COMMENTARY Intrinsic Signals and Functional Brain Mapping: Caution, Blood Vessels at Work Costantino Iadecola It has long been thought that there is a close relationship between brain activity and cerebral blood f low [reviewed by Raichle (Raichle, 1998)]. While in 1890 Roy and Sherrington proposed the concept of an ‘intrinsic mechanisms’ responsible for coupling neural activity to blood f low (Roy and Sherrington, 1890), more than a decade earlier the Italian physiologist Angelo Mosso was already undertaking a detailed study of the changes in cerebral hemodynamics associated with various psychological states in humans (Mosso, 1881). Following up on these pioneering efforts, our understanding of the localization of brain function has advanced considerably thanks to techniques that detect the changes in brain blood f low associated with brain activity (Table 1). One of the most recent approaches is based on monitoring activity-induced increases in cerebral oxygenation. During functional activation cerebral blood f low increases more than the oxygen demands of the brain (Fox and Raichle, 1986), and such mismatch results in an increase in oxyhemoglobin and a decrease in deoxyhemoglobin (Raichle, 1998). Activityinduced increases in brain oxygenation can be detected either by magnetic resonance imaging (MRI), as blood oxygenation level dependent (BOLD) contrast (Ogawa et al., 1992), or, by optical imaging, as ‘intrinsic signals’ (Grinvald et al., 1986). Because of the importance and widespread use of these approaches in functional brain mapping, there is a great interest in elucidating the precise mechanisms by which these signals are generated and how accurately they ref lect brain activity (Raichle, 1998). In the present issue of Cerebral Cortex, Harrison and colleagues (Harrison et al., 2002) provide a much-needed piece of information concerning the relationship between intrinsic signals and cerebral vasculature. These authors used optical imaging to investigate the intrinsic signals evoked by auditory stimuli in the auditory cortex of the chinchilla. The spatial distribution of the signals was then correlated with the distribution of cerebral blood vessels, visualized in the same animal post mortem by corrosion casts. Through this careful analysis they were able to determine that the area in which intrinsic signals are detected overlaps remarkably well with the distribution of cerebral blood vessels supplying the activated region. Thus, signals emanated only from areas endowed with a rich vascular network, and no signals were obtained from adjacent regions in which the vasculature was less dense. Although neural activity was not mapped in the same animals in which the optical recordings and vascular casts were obtained, a map correlating unit activity with intrinsic signals in separate animals revealed that there was a general correspondence between active sites and location of the intrinsic signals. However, in some cases unit activity was not detected in areas in which intrinsic signals were generated. They also found that the cerebral vasculature is endowed with sphincter-like structures, often strategically placed at arteriolar branch points. The authors argue that these structures are involved in controlling the local distribution of f low within the vascular network. These findings have notable implications for functional brain mapping using hemodynamic changes as a ‘proxy’ for neural activity. On the one hand, the finding that intrinsic signals identif y reasonably well the area of activation, assessed by electrophysiological recordings, supports the validity of using vascular-based methods to localize brain function. On the other hand, the observation that the topography of the intrinsic signals overlaps with the distribution of cerebral blood vessels within the activated region suggests that the vascular geometry of a brain region dictates the spatial distribution of these signals. Therefore, depending on the spatial relationships between vascular network and active neurons, there might be instances in which the area of activation will not match the area from which the signals are generated. This point is demonstrated by the data of Harrison et al. (Fig. 2) showing that intrinsic signals can be detected from areas in which there is no evoked neural activity. Although in some areas vascular and neural architecture are well matched, e.g. in the rodent whisker–barrel cortex (Woolsey et al., 1996), this correspondence cannot be assumed to occur in all brain regions and for all activation paradigms. Another factor that may prevent complete overlap between vascular and activity maps is that the vascular dilatation responsible for the increase in blood f low evoked by neural activity is propagated in a retrograde fashion to upstream arterioles located outside the activated area (Iadecola et al., 1997). This phenomenon, termed retrograde vasodilation, serves the purpose of dilating upstream arterioles that are critical for regional f low control. Therefore, during functional activation, an increase in f low is observed also in remote regions in which no neural activity can be detected. Another implication of the data of Harrison et al. is that the intensity of the intrinsic signals generated from a brain region depends on local vascular density. Therefore, given an equal degree of neural activation, the area in which vascular density is more sparse will generate a less robust intrinsic signal. Consequently, the ‘hot spots’ on a functional map may not necessarily indicate the areas that are most active in the execution of a specific task. While Harrison et al. focused on the relationship between intrinsic signals and vascular networks, Logothetis and colleagues investigated the specific components of the neural activity linked to the vascular signal (Logothetis et al., 2001). These authors recorded simultaneously electrophysiological parameters and BOLD fMRI in the monkey visual cortex and found that field potentials, rather than spiking activity, best predicted the vascular signal. This finding suggests that neural input and local processing, rather than neural output, are the most important determinants of the vascular change, a conclusion reached also by others on the basis of direct f low measurements in cerebellum (Mathiesen et al., 1998). © Oxford University Press 2002. All rights reserved. Cerebral Cortex Mar 2002;12:223–224; 1047–3211/02/$4.00 Department of Neurology, University of Minnesota, Minneapolis, MN 55455, USA Table 1 Historical overview of activity-induced cerebrovascular changes in the human brain Authors Finding Technique (Mosso, 1881) (Fulton, 1928) (Kety, 1950) Increased brain volume during mental processing Increased flow turbulence in occipital cortex during visual stimulation Increase in flow and cerebral metabolic rate for oxygen during anxiety (Ingvar and Risberg, 1967) (Fox and Raichle, 1986) Increased cerebral blood flow in speech-related areas during word repetition Sensory stimulation increases blood flow in somatosensory cortex out of proportion to oxygen use Increased blood volume in visual cortex during photic stimulation Increased blood oxygenation level dependent (BOLD) contrast in visual cortex during photic stimulation Monitoring of intracranial pressure through a breach in the skull Recording of bruit from vascular malformation in occipital cortex Cerebral artero-venous difference of inhaled nitrous oxide (Kety and Schmidt method) Regional brain clearance of 133Xe injected into the carotid artery Positron emission tomography using 15O water as a tracer (Belliveau et al., 1991) (Ogawa et al., 1992) Logothetis and colleagues also point out that, due to its higher signal-to-noise ratio, neural activity can be present in areas in which no BOLD contrast is detected, resulting in underestimation of activated areas assessed by fMRI. This conclusion also suggests caution in the functional interpretation of activity maps based exclusively on the presence or absence of the BOLD signal. The finding that there might be sphincter-like structures capable of controlling the distribution of f low within the cerebral microvascular network is also of interest, and its significance has to be discussed in the context of a large body of work suggesting that cerebral blood vessels receive neural projections from intrinsic neurons (Eckenstein and Baughman, 1984; Vaucher and Hamel, 1995; Krimer et al., 1998). It is tempting to speculate that these sphincter-like structures are controlled by neural projections originating from neurons dedicated to f low regulation. Such neurovascular units would be well suited to control the timing and spatial distribution of the increases in f low evoked by neural activity, and could regulate f low for purposes other than to support the changing energetic needs of the tissue. For example, these neural networks could mediate anticipatory increases in blood f low in preparation for, rather than in response to, an increase in energy demands. Such anticipatory neurovascular control could, perhaps, explain why during activation the increase in cerebral blood f low greatly exceeds the oxygen needs of the tissue. This hypothesis, however, awaits experimental confirmation. Notes Address correspondence to Costantino Iadecola, Department of Neurology, University of Minnesota, 516 Delaware Street SE, Minneapolis, MN 55455, USA.Email: [email protected]. References Belliveau JW, Kennedy DN, McKinstry RC, Buchbinder BR, Weisskoff RM, Cohen MS, Vevea JM, Brady TJ, Rosen BR (1991) Functional mapping of the human visual cortex by magnetic resonance imaging. Science 254:716–719. Eckenstein F, Baughman R (1984) Two types of cholinergic innervation in the cortex, one colocalized with vasoactive intestinal polypeptide. Nature 309:153–155. Fox PT, Raichle ME (1986) Focal physiological uncoupling of cerebral 224 Intrinsic Signals and Functional Brain Mapping • Iadecola MRI using a paramagnetic contrast agent injected intravenously MRI using intrinsic signals deriving from deoxyhemoglobin blood f low and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA 83:1140–1144. Fulton JF (1928) observations upon the vascularity of the human occipital lobe during visual activity. Brain LI:310–320. Grinvald A, Lieke E, Frostig RP, Gilbert C, Weisel TM (1986) Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature 324:361–364. Harrison RV, Harel N, Panesar J, Mount RJ (2002) Blood capillary distribution correlates with hemodynamic-based functional imaging in cerebral cortex. Cereb Cortex 12: 225–233. Iadecola C, Yang G, Ebner T, Cheng G (1997) Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol 78:651–659. Ingvar DH, Risberg J (1967) Increase of regional cerebral blood f low during mental effort in normals and in patients with focal brain disorders. Exp Brain Res 3:195–211. Kety SS (1950) Circulation and metabolism of the human brain in health and disease. Am J Med 8:205–217. Krimer LS, Muly EC, 3rd, Williams GV, Goldman–Rakic PS (1998) Dopaminergic regulation of cerebral cortical microcirculation. Nature Neurosci 1:286–289. Logothetis NK, Pauls J, Augath M, Trinath T, oeltermann A (2001) Neurophysiological investigation of the basis of the fMRI signal. Nature 412:150–157. Mathiesen C, Caesar K, Akgoren N, Lauritzen M (1998) Modification of activity-dependent increases of cerebral blood f low by excitatory synaptic activity and spikes in rat cerebellar cortex. J Physiol 512:555–566. Mosso A (1881) Ueber den Kreislauf des Blutes im menschlichen Gehirn. Leipzig: Viet. Ogawa S, Tank DW, Menon R, Ellerman JM, Kim S-G, Merkle H, Ugurbil. K (1992) Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA 89:5951–5955. Raichle ME (1998) Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci USA 95:765–772. Roy C, Sherrington C (1890) on the regulation of the blood-supply of the brain. J Physiol 11:85–108. Vaucher E, Hamel E (1995) Cholinergic basal forebrain neurons project to cortical microvessels in the rat: electron microscopic study with anterogradely transported Phaseolus vulgaris leucoagglutinin and choline acetyltransferase immunocytochemistry. J Neurosci 15:7427–7441. Woolsey TA, Rovainen CM, Cox SB, Henegar MH, Liang GE, Liu D, Moskalenko Y E, Sui J, Wei L (1996) Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cereb Cortex 6:647–660.