* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Molecular architecture of the glomerular slit
Survey
Document related concepts
Protein folding wikipedia , lookup
Circular dichroism wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Protein domain wikipedia , lookup
Protein purification wikipedia , lookup
Protein moonlighting wikipedia , lookup
Trimeric autotransporter adhesin wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Western blot wikipedia , lookup
Transcript
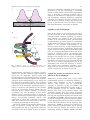
Nephrol Dial Transplant (2007) 22: 2124–2128 doi:10.1093/ndt/gfm344 Advance Access publication 5 June 2007 Editorial Review Molecular architecture of the glomerular slit diaphragm: lessons learnt for a better understanding of disease pathogenesis Harry Holthöfer National Center of Sensor Research/BioAnalytical Sciences, Dublin City University, Ireland Keywords: cell-adhesion molecules; intercellular junctions; proteinuria; podocyte Introduction For more than three decades, the molecular composition of the interpodocyte slit diaphragm of the glomerular filtration barrier has remained elusive. The first electron microscopic studies described the slit diaphragm as a porous, ‘zipper-like’ structure [1], but it was not until 1998 that the first transmembrane molecule of the slit diaphragm was identified [2]: Nephrin is a cell surface receptor of the immunoglobulin superfamily participating in cell–cell adhesion and signalling functions [3]. Mutations in nephrin lead to the congenital nephrotic syndrome of the Finnish type (CNF), suggesting that nephrin is of pivotal importance for maintaining the filtration barrier. In recent years, the mapping of the genetic background of other inherited and acquired nephropathies and the generation of transgenic animal models have led to the beginning of a new era in nephrology, also promising new targeted therapies and advanced diagnostics. In the present review, the main recent findings exploring the molecular architecture of the glomerular filter itself and their role in proteinuric glomerular diseases are reviewed. The slit diaphragm of the glomerular capillary wall Proteinuria is the hallmark of glomerular damage. While there is strong evidence that even a minute loss of albumin in urine reflects a first perturbation of the glomerular filtration barrier, there also appears to be a direct link between proteinuria and vascular damage. Thus, proteinuria is a risk factor for both progressive Correspondence and offprint requests to: Harry Holthöfer, MD, PhD, Biomedicum Helsinki, University of Helsinki, PB 63, FI-00014, Finland. Email: [email protected] renal disease and cardiovascular disease [4]. Either the protein leakage through the glomerular capillary wall itself, or direct spreading of glomerular damage to the extraglomerular space appears to lead to a sequence of downstream events including interstitial inflammation and, finally, the loss of nephron function [5]. Podocytes interlinked by the slit diaphragms constitute a continuous outermost layer in the glomerular filtration barrier (Figure 1). This barrier has size- and charge-selective properties, and the loss (internalization) of the slit diaphragm associates with disruption of the filter, as reflected by proteinuria [6]. The exact mechanisms and causalities remain to be described in detail. Rodewald and Karnovsky [1] suggest that the slit diaphragm is a zipper-like structure that functions as a sieve, with a pore size smaller than albumin. Recent studies using electron tomography have suggested that, like many other biologic membranes, the slit diaphragm is composed of an organized network of winding strands, possibly constituting the base into which various molecular components are intertwined. Since the slit diaphragm appears as the final seal to prevent loss of circulating proteins into urine, this unique intercellular junction has been the target for increasing research interest. At the molecular level, the slit diaphragm has been referred to as a modified ‘adherens junction’ by Reiser et al. [7]. Indeed, this junctional complex has components typical for tight junctions (ZO-1) [8] as well as adherens junctions (P-cadherin [9]). Nephrin forms the scaffold for intertwining slit molecules Congenital nephrotic syndrome of the Finnish type (CNF) is an autosomal recessive disorder, characterized by massive proteinuria and nephrosis already in utero [10]. The process that begun from the first descriptions of this rare, but severe paediatric disease led to the final cloning of the causative NPHS1 gene, in 1998 [2]. The gene product of NPHS1, nephrin, is a type-1 transmembrane protein belonging to the ß The Author [2007]. Published by Oxford University Press on behalf of ERA-EDTA. All rights reserved. For Permissions, please email: [email protected] Molecular architecture of the glomerular slit diaphragm 2125 Injection of antinephrin antibodies results in massive proteinuria in rats [18]. Interestingly, a substantial amount of CNF patients treated with transplantation show a recurrence of nephrotic syndrome due to induction of autoimmunity to nephrin in the transplant and subsequently emerging circulating antinephrin antibodies [19]. Nephrin has been proposed as an early marker of podocyte damage [20], reflecting the patency of the filtration barrier even more accurately than microalbuminuria, particularly in diabetes. A Urinary space Slit diaphragm Podocyte foot process Glomerular basement membrane Endothelium Signalling at the slit diaphragm B Urinary space ZO-1 Filtrin Podocyte foot process NEPH1/2 Slit diaphragm Nephrin Podocin Actin Fyn CD2AP FAT Beta-catenin P-cadherin Densin Alpha-actinin-4 Synaptopodin Fig. 1. Schematic picture of podocyte architechture (A). In (B), key slit area molecules and their interlinkages are shown. immunoglobulin superfamily of adhesion proteins. Nephrin was the first identified integral membrane protein the slit diaphragm. Several studies have provided support for the hypothesis that the outermost N-terminal Ig-like domains of nephrin from aadjacent podocytes interact homotypically [11,12], linking the foot processes dynamically together. This interaction most likely provides the structural but also functional scaffold for the slit diaphragm. Nephrin has shown to interact in podocytes with CD2AP [13] and podocin [14] via its intracellular domain, linking the slitassociated protein complex ultimately to podocyte cytoskeleton [15]. The interactions of this protein complex with the cytoskeleton appear to provide the basis for translating extracellular events to rapid shape changes characterizing proteinuric diseases. The mechanisms by which this is achieved are presently under active research and could provide a future site for targeted pharmacological interventions. Expression of nephrin is regulated at different levels: usage of alternative splicing [16] and a naturally occurring antisense gene [17], supposedly needed for fine-tuning of the tissues-specific presence of nephrin. Since the first reports, it has become clear that one of the basic functions of the slit diaphragm-associated molecules include outside-in signalling. In nephrin, these functions are enabled by the nine tyrosine residues of the intracellular domain, phosphorylated at ligand binding [21]. Other ligands for extracellular nephrin remain to be discovered but obviously offer, in addition to the molecular understanding of the filtration barrier machinery, another intriguing future site of targeted therapies. Interestingly, oligomerized nephrin associates with signaling microdomains, lipid rafts, in a cholesterol-dependent manner [22]. In-vivo injection of antibodies against 9-O-acetylated GD3 ganglioside of podocytes led to rapid morphological changes at the filtration slit area, closely resembling foot process effacement. At the same time, nephrin dislocated to the apical pole of the narrowed filtration slits and was tyrosine phosphorylated [22]. It has subsequently been shown that clustering of extracellular nephrin with antinephrin antibodies in-vitro disrupts cell–cell contacts [23] and leads to phosphorylation of nephrin by Src family kinases [24]. Nephrin-like proteins are essential for cell–cell adhesion at the slit diaphragm Nephrin-like proteins include three closely related transmembrane proteins of substantial similarity to nephrin which contribute to the slit diaphragm structure. Like nephrin, they are preferentially expressed in podocytes [25,26], and NEPH1 and NEPH2 have been determined to localize closely to the slit diaphragm [12,27]. In addition, NEPH1deficient mice suffer from perinatal lethality and proteinuria together with foot process effacement [28], thus resembling the findings in nephrin-deficient mice [29]. The extracellular nephrin core interacts both with NEPH1 and NEPH2, whereas they do not appear to interact homotypically with each other like nephrin [12,30]. Nephrin-like proteins also interact with ZO-1, the first identified molecule of the slit diaphragm area. Interestingly, NEPH2 is cleaved from podocytes by locally activated metalloproteinases and is released into the urine [27]. Podocin also interacts with the intracellular domains of NEPH1, NEPH2 and filtrin 2126 [25] suggesting that it is a shared linker for many of the essential slit diaphragm molecules. P-cadherin and FAT1 localize to the slit diaphragm P-cadherin may contribute to the molecular complex with defined functions for the maintenance of the filtration slit barrier [31]. However, since P-cadherin deficient mice and humans with mutation in the P-cadherin gene show no kidney phenotype, it obviously does not have a direct role in glomerular filtration. FAT1 is a distinct member of the cadherin superfamily of adhesion molecules and has been localized to the slit area, co-localizing with nephrin as well as with ZO-1 [32]. Mice lacking FAT1 are born with effaced foot processes without slit diaphragms and die within 48 h post-natally [33]. FAT1 is possibly needed for the intercellular adhesion framework between foot processes, while at the same time severing the intercellular gap distance; as supported by findings in the FAT1 knock-out model [33], this molecule is apparently centrally involved in the organizaation of actin dynamics. Intracellular scaffold and adaptor proteins of podocytes CD2-associated protein (CD2AP) was initially described in the T-lymphocyte CD2 protein complex [13,34], but it is also present in the slit diaphragm area. It links intracellular nephrin by the C-terminal domain. Importantly for the signalling function of the complex, the N-terminal part of CD2AP potentiates nephrin-induced AKT-mediated signalling [34]. The CD2AP-knockout mice present with a severe kidney phenotype and defects in foot process formation [34]. CD2AP and nephrin may mediate their functions via connections to the actin cytoskeleton, as evidenced by the shape changes typical for the podocyte in proteinuria. Podocin was initially discovered in autosomal recessive and in sporadic familiar focal segmental glomerulosclerosis patients [14]. Podocin-knockout mice also show antenatal proteinuria, foot process effacement and reduced nephrin expression [35]. Podocin is an integral membrane protein and, unlike nephrin, appears strictly localized in podocytes. Podocin binds by its C-terminal domain with CD2AP and nephrin to form a scaffolding complex of the slit diaphragm, but also enhances the mitogen-activated protein kinase cascades by recruiting nephrin into lipid rafts [36,37] and the subsequent signalling. Thus, these two molecules appear to be functionally strongly interacting. Podocin also interacts with the intracellular domains of NEPH1, NEPH2 and filtrin [25], suggesting that it is the shared linker for many of the essential slit diaphragm molecules. H. Holthöfer a-Actinin-4 is a cytosolic actin-filament cross-linking protein. In autosomal dominant familial focal segmental glomerulosclerosis, a-actinin-4 is typically mutated [38], suggesting that a-actinin-4 is a robust linker molecule, mediating and modulating interactions of the slit diaphragm domain molecules with the actin cytoskeleton of podocytes. Densin is a heavily sialylated post-synaptic protein of the brain. Interestingly, it is among the growing group of molecules only shared with the podocyte and the neurons [39]. Densin interacts either directly, or possibly via some as yet unrecognized intermediates, with nephrin and localizes strictly to the slit diaphragm area. Interestingly, densin mRNA and protein expression is increased in CNF kidneys [39], suggesting that its expression may account for compensatory mechanisms in kidney damage. Conclusion While being a key element in the glomerular filtration barrier and a direct target for damages in hypertension, diabetes and other proteinuric diseases, the podocyte, and particularly the slit diaphragm, have remained poorly understood cells. They share remarkable morphological and molecular similarities with neurons and cells of the immunological systems. They are terminally differentiated, with extremely tight growth control, being virtually the only cell type in the body without known malignant transformation. Few pharmaceutically effective molecules have been available for treating diseases deriving from podocyte dysfunction and its consequences. With the milestone discovery of nephrin, its cell biology and the avalanche of subsequent molecular components maintaining the glomerular filtration barrier, we are rapidly entering a new era identifying a set of pharmacologic targets. Will the new predictive diagnostics and therapies be developed fast enough to combat the avalanche of chronic kidney disease so often starting from podocyte dysfunction? Acknowledgements. Figure 1 is courtesy of Johanna RintaValkama (University of Helsinki) which is gratefully acknowledged. Conflict of interest statement. None declared. References 1. Rodewald R, Karnovsky MJ. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 1974; 60: 423–433 2. Kestila M, Lenkkeri U, Mannikko M et al. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell 1998; 1: 575–582 3. Lenkkeri U, Mannikko M, McCready P et al. Structure of the gene for congenital nephrotic syndrome of the finnish type (NPHS1) and characterization of mutations. Am J Hum Genet 1999; 1: 51–61 Molecular architecture of the glomerular slit diaphragm 4. Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 2006; 2: 288–296 5. Kriz W, LeHir. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 2005; 67: 404–419 6. Tryggvason K. Unraveling the mechanisms of glomerular ultrafiltration: nephrin, a key component of the slit diaphragm. J Am Soc Nephrol 1999; 10: 2440–2445 7. Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 2000; 11: 1–8 8. Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol 1990; 111: 1255–1263 9. Balda MS, Matter K. Transmembrane proteins of tight junctions. Semin Cell Dev Biol 2000; 11: 281–299 10. Hallman N, Hjelt L, Ahvenainen EK. Nephrotic syndrome in newborn and young infants. Annales Paediatriae Fenniae 1956; 2: 227–241 11. Gerke P, Huber TB, Sellin L, Benzing T, Walz G. Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and NEPH1. J Am Soc Nephrol 2003; 14: 918–926 12. Barletta GM, Kovari IA, Verma RK, Kerjaschki D, Holzman LB. Nephrin and Neph1 Co-localize at the Podocyte Foot Process Intercellular Junction and Form cis Heterooligomers. J Biol Chem 2003; 278: 19266–19271 13. Shih NY, Li J, Cotran R, Mundel P, Miner JH, Shaw AS. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol 2001; 159: 2303–2308 14. Boute N, Gribouval O, Roselli S et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 2000; 24: 349–354 15. Yuan H, Takeuchi E, Taylor GA, McLaughlin M, Brown D, Salant DJ. Nephrin dissociates from actin, and its expression is reduced in early experimental membranous nephropathy. J Am Soc Nephrol 2002; 13: 946–956 16. Holthofer H, Ahola H, Solin ML et al. Nephrin localizes at the podocyte filtration slit area and is characteristically spliced in the human kidney. Am J Pathol 1999; 155: 1681–1687 17. Ihalmo P, Rinta-Valkama J, Mai P et al. Molecular cloning and characterization of an endogenous antisense transcript of Nphs1. Genomics 2004; 83: 1134–1140 18. Orikasa M, Matsui K, Oite T, Shimizu F. Massive proteinuria induced in rats by a single intravenous injection of a monoclonal antibody. J Immunol 1988; 141: 807–814 19. Wang SX, Ahola H, Palmen T, Solin ML, Luimula P, Holthofer H. Recurrence of nephrotic syndrome after transplantation in CNF is due to autoantibodies to nephrin. Exp Nephrol 2001; 9: 327–331 20. Pätäri A, Forsblom C, Havana M et al. Nephrinuria in Diabetic Nephropathy of Type 1 Diabetes. Diabetes 2003; 52: 2969–2674 21. Verma R, Wharram B, Kovari I et al. Fyn binds to and phosphorylates the kidney slit diaphragm component nephrin. J Biol Chem 2003; 278: 20716–20723 22. Simons M, Schwarz K, Kriz W et al. Involvement of lipid rafts in nephrin phosphorylation and organization of the glomerular slit diaphragm. Am J Pathol 2001; 159: 1069–1077 23. Khoshnoodi J, Sigmundsson K, Ofverstedt LG et al. Nephrin Promotes Cell-Cell Adhesion through Homophilic Interactions. Am J Pathol 2003; 163: 2337–2346 24. Lahdenperä J, Kilpeläinen P, Liu XL et al. Clustering-induced tyrosine phosphorylation of nephrin by Src family kinases. Kidney Int 2003; 64: 404–413 25. Sellin L, Huber TB, Gerke P, Quack I, Pavenstadt H, Walz G. NEPH1 defines a novel family of podocin interacting proteins. FASEB J 2003; 17: 115–117 2127 26. Ihalmo P, Palmen T, Ahola H, Valtonen E, Holthofer H. Filtrin is a novel member of nephrin-like proteins. Biochem Biophys Res Commun 2003; 300: 364–370 27. Gerke P, Sellin L, Kretz O et al. NEPH2 is located at the glomerular slit diaphragm, interacts with nephrin and is cleaved from podocytes by metalloproteinases. J Am Soc Nephrol 2005; 16: 1693–1702 28. Donoviel DB, Freed DD, Vogel H et al. Proteinuria and perinatal lethality in mice lacking neph1, a novel protein with homology to nephrin. Mol Cell Biol 2001; 21: 4829–4836 29. Rantanen M, Palmen T, Patari A et al. Nephrin TRAP Mice Lack Slit Diaphragms and Show Fibrotic Glomeruli and Cystic Tubular Lesions. J Am Soc Nephrol 2002; 13: 1586–1594 30. Liu G, Kaw B, Kurfis J, Rahmanuddin S, Kanwar YS, Chugh SS. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest 2003; 112: 209–221 31. Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 2000; 11: 1–8 32. Inoue T, Yaoita E, Kurihara H et al. FAT is a component of glomerular slit diaphragms. Kidney Int 2001; 59: 1003–1012 33. Ciani L, Patel A, Allen ND, ffrench-Constant C. Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol Cell Biol 2003; 23: 3575–3582 34. Shih NY, Li J, Karpitskii V et al. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 1999; 286: 312–315 35. Roselli S, Heidet L, Sich M et al. Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol Cell Biol 2004; 24: 550–560 36. Huber TB, Kottgen M, Schilling B, Walz G, Benzing T. Interaction with podocin facilitates nephrin signaling. J Biol Chem 2001; 276: 41543–41546 37. Huber TB, Simons M, Hartleben B et al. Molecular basis of the functional podocin-nephrin complex: mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Human Mol Genet 2003; 12: 3397–3405 38. Kaplan JM, Kim SH, North KN et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 2000; 24: 251–256 39. Ahola H, Heikkilä E, Åström E et al. A novel protein, densin, expressed by glomerular podocytes. J Am Soc Nephrol 2003; 14: 1731–1737 40. Winn MP, Conlon PJ, Lynn KL et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 2005; 308: 1801–1804 41. Takeda T. Podocyte cytoskeleton is connected to the integral membrane protein podocalyxin through Naþ/Hþ exchanger regulatory factor 2 and ezrin. Clin Exp Nephrol 2003; 7: 260–269 42. Thomas PE, Wharram BL, Goyal M et al. GLEPP1, a renal glomerular epithelial cell (podocyte) membrane protein-tyrosine phosphatase. J Biol Chem 1994; 269: 19953–19962 43. Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 2003; 83: 253–307 44. Raats CJ, van den Born J, Bakker MA et al. Expression of agrin, dystroglycan and utrophin in normal renal tissue and in experimental glomerulopathies. Am J Pathol 2000; 156: 1749–1765 45. Regoli M, Bendayan M. Alterations in the expression of the alpha3 beta 1 integrin in certain membrane domains of the glomerular epithelial cells (podocytes) in diabetes mellitus. Diabetologia 1997; 40: 15–22 46. Harita Y, Miyauchi N, Karasawa T et al. Altered expression of junctional adhesion molecule 4 (JAM4) in injured podocytes. Am J Physiol Renal Physiol 2005 2128 47. Kerjaschki D, Farquhar MG. Immunocytochemical localozation of Heymann nephritis antigen (gp330) in glomerular epithelial cells of normal Lewis rats. J Exp med 1983; 157: 667–686 48. Patrie KM, Drescher AJ, Goyal M, Wiggins RC, Margolis B. The membrane associated guanylate kinase protein MAGI-1 H. Holthöfer binds megalin and is present in glomerular podocytes. J Am Soc Nephrol 2001; 12: 667–677 49. Rastaldi MP, Armelloni S, Berra S et al. Glomerular podocytes contain neuron-like functional synaptic vesicles. FASEB J 2006; 20: 976–978 Received for publication: 25.10.06 Accepted in revised form: 4.5.07