* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1) Discuss if NOCICEPTORS are real. 2) Describe the distribution of
Multielectrode array wikipedia , lookup
Neuroregeneration wikipedia , lookup
Perception of infrasound wikipedia , lookup
Psychophysics wikipedia , lookup
Axon guidance wikipedia , lookup
Neural coding wikipedia , lookup
Biological neuron model wikipedia , lookup
Electrophysiology wikipedia , lookup
Neurotransmitter wikipedia , lookup
Nervous system network models wikipedia , lookup
End-plate potential wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Sensory substitution wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Development of the nervous system wikipedia , lookup
Synaptogenesis wikipedia , lookup
Neuroanatomy wikipedia , lookup
Central pattern generator wikipedia , lookup
Synaptic gating wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Optogenetics wikipedia , lookup
Signal transduction wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Circumventricular organs wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Microneurography wikipedia , lookup
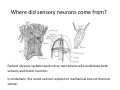
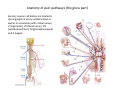
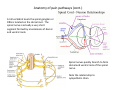
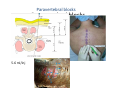
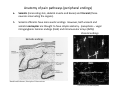
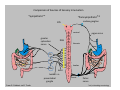
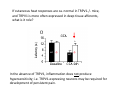
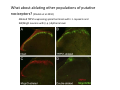
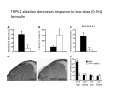
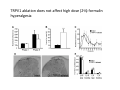
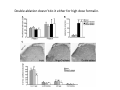
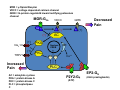
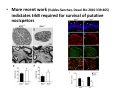
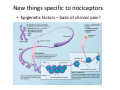
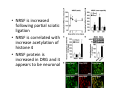
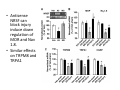
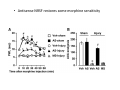
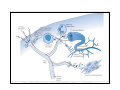
Goals for this lectures on structure and function of “NOCICEPTORS” 1) Discuss if NOCICEPTORS are real. 2) Describe the distribution of sensory neurons and their relationship to peripheral anatomical structures that might affect their function 3) Describe chemical heterogeneity of “nociceptors” 4) Discuss potential functions of “nociceptors” as afferent and efferent neurons and what they might do while they are waiting around for you to touch the stove. There is no spoon: It’s a nociceptor My problem: • Why would the mammalian body devote at least 50% of all sensory neurons to nociception? • What are these guys during until you step on a nail? • How often would the evolving animals encounter noxious heat? • Why would nociceptive molecules that can detect specific noxious stimuli be expressed in tissues that are not exposed to those stimuli (e.g. TRPV1 in muscle). • For visceral afferents, what’s a caveman going to do about pancreatitis, appendicitis, IBS? My Problem (cont.) – Bias in science From “Evolutionary aspect of Pain” by ET Walters Additional species, including snails (Malyshev, A. Y. and Balaban, P. M., 2002) and lampreys (Christenson, J. et al., 1988) have mechanosensory neurons that are likely to be nociceptive, despite their relatively low mechanical thresholds, because the tissue they innervate is delicate enough to be damaged by modest pressures. Paraphrased from “Nociceptors: a phylogenetic view” (J. Comp Phys 2009) Smith and Lewin “In aplysia, the first potential nociceptors were identified in the abdominal ganglion but these were originally identified as being low threshold mechanoreceptors. However, if pinned out correctly these siphon innervating cells were shown to have high threshold with maximal activity occurring when crushing/tearing stimuli, causing body wall damage were used.” “When they do not consider the role of evolution in their hypotheses, scientists are no better than the taliban” (BMD 2011) Where did sensory neurons come from? Earliest nervous systems were nerve nets where cells could have both sensory and motor function. In cnidarians, the nerve net can respond to mechanical but not thermal stimuli. What were nociceptor’s evolutionary competition/source? • Primitive vertebrates had – Visual organs – Olfactory organs – Low threshold mechanoreceptors – Electroreceptors – Lateral line organs – Magnetoreceptors – Chemoreceptors What properties would you include in a professional nociceptor • Function – must code noxious stimuli • Anatomy – must connect to higher centers that contribute to pain sensations • Commitment – does this have to be their primary job? • Can be sensitized (early form of learning?) • If ablated, pain is gone • MUST BE A C‐FIBER (sharks don’t have C‐fibers) Other possible roles for “nociceptors” • As efferents – Regulating vascular tone via local reflexes – Modifying immune interactions via stimulation of resident immune cells – Contributing to homeostasis of targets (receptors for virtually all sensory neuron transmitters are present in the periphery) • As afferents – Detectors endogenous and exogenous substances (e.g. endogenous cannabinoids, food related substances) – Low threshold stimuli (for “nociceptors with wdr). From Sherrington Lectures 1906 • “…there should exist a subpopulation of somatosensory neurons, called nociceptors, which are activated only by stimuli intense enough to threaten or cause tissue damage.” Stained glass window in the dining hall of Gonville and Caius College, in Cambridge (UK), From Wikipedia • A nociceptor is a sensory receptor that responds to potentially damaging stimuli by sending nerve signals to the spinal cord and brain. This process, called nociception, usually causes the perception of pain. From IASP website • Nociceptor A receptor preferentially sensitive to a noxious stimulus or to a stimulus which would become noxious if prolonged. Note: Avoid use of terms like pain receptor, pain pathway, etc*. *The terms to avoid were originally suggested by Sherrington since these cells could produce reflexes in spinal animals that could not detect “pain”. Anatomy of sensory neurons a. Sensory neurons are defined as those neurons that collect information regarding either the external or internal environment, b. Sensory neurons including nociceptors are pseudounipolar neurons. They do NOT have well‐defined axons or dendrites. They have central and peripheral processes. c. Nociceptors would be a special class of sensory neurons with specific properties Soma Peripheral process Central process Target Dorsal horn Anatomy of pain pathways (the gross part) Sensory neuron cell bodies are located in spinal ganglia at every vertebral level as well as in association with cranial nerves, V (trigeminal), VII (facial nerve), VIII (vestibulocochlear), IX (glossopharyngeal) and X (vagus). Anatomy of pain pathways (cont.) At all vertebral levels the spinal ganglion or DRG is located on the dorsal root. The spinal nerve is actually a very short segment formed by anastomosis of dorsal and ventral roots. Spinal nerves quickly branch to form dorsal and ventral rami of the spinal nerve. Note the relationship to sympathetic chain. Paravertebral blocks Paravertebral blocks 5‐6 ml/inj Anatomy of pain pathways (peripheral endings) a. Somatic (innervating skin, skeletal muscle and bones) and Visceral (those neurons innervating the organs). b. Somatic afferents have more exotic endings. However, both visceral and somatic nociceptor are thought to have simple anatomy. (exceptions – vagal intraganglionic laminar endings (IGLE) and intramuscular arrays (IMA)) Somatic endings Visceral endings IGLE IMA Kandel and Schwartz, Principles of Neuroscience From Fox et al., JCN 428:558 (2000) Sherrington proposed that nociceptors had “naked” endings • Sherrington knew that the corneal stimulation produced exclusively painful sensations. • He also new that stimuli applied directly to nerve –not endings‐ could also induce responses. • Concluded that observed corneal fibers must be nociceptors and suggested that this anatomy was shared by nociceptors everywhere. From Peter Lwigale, Rice University. Chick cornea Afferent Fibers a) b) c) d) Peripheral sensory fibers come in three sizes: C, Aδ and Aβ. C‐fibers are unmyelinated and are the most common (conduction velocity < 2m/s). Aδ (CV < 4‐10m/s rodents, <30m/s primates) are lightly myelinated whereas Aβ (CV >4‐ 10m/s rodents, >30m/s primates) are heavily myelinated. “NOCICEPTORS” COME IN ALL THREE SIZES!!!! (although visceral nociceptors are 10 C‐ fibers with some Aδ). C‐fibers Aδ Schwann cell nucleus Aβ From Louisa Howard and Michael Binder, Dartmouth Many Aβ fibers are “nociceptors” Species AB HTM/A‐nociceptors (%) Monkey 18 Cat 38 Cat 30 Cat 22 Rat (adult) ca 48 Rat (5 wk) ca. 65 Rat (7wk) 64 Guinea Pig (1‐2 wk) 48 Guinea Pig (1‐3wk) 50 Mouse (adult) 25% >10m/s 50% > 7m/s (from Djouhri and Lawson, 2004 ‐ one your handouts) Somatic nociceptors central terminations Top panel: Two types of non‐ nociceptive endings. Note symmetrical nature of spike shape. Bottom panel: Two nociceptors ‐ one myelinated (HTMR) and one C‐fiber. Note similarity of spike shape (broad with inflection on falling phase). Non‐nociceptive C‐fibers also have this shape but non‐nociceptive A‐ fibers do not. Also note that nociceptors terminate more dorsally than non‐nociceptors. From Koerber and Woodbury, 2002 Not all nociceptors have discrete projections Visceral afferent have very large central projections Reconstruction of an intracellularly filled visceral afferent (Sugiura et al, J. Neurophysiol. 1989 62:834). Peripheral Visceral Pain pathways a) Visceral sensations are carried in two different pathways. b) Both are associated with the autonomic nervous system, but they are NOT part of it. c) The most substantial of these runs with the vagus nerve that has superior and inferior sensory ganglia d) Dogma – vagal afferents contribute only to affective dimensions of pain. Cranial nerve X ‐ Vagus Visceral Pain Pathways (cont) a) The second visceral pain pathway begins in spinal ganglia. b) The axons from these afferents run with the sympathetic preganglionic neurons to reach the abdominal and pelvic organs. c) They are NOT part of the sympathetic nervous system. d) Activity from these neurons does reach consciousness. e) The prevertebral ganglia associated with this pathway are favorite targets for nerve blocks (e.g. celiac ganglion). Comparison of Sources of Sensory Innervation “Sympathetic”* “Parasympathetic”* nodose ganglion NTS greater splanchnic nerve DRG CG SMG IMG least lumbar s.n. prevertebral ganglia From G. Gebhart via R. Traub 1 2 3 4 5 6 7 8 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3 4 5 1 2 3 4 5 cervical vagus nerve thoracic lumbar sacral Pelvic nerve *evil, misleading terminology Nociceptor neurotransmitters a) Sensory neurons are also heterogeneous in terms of the neurotransmitters they release both centrally and peripherally. b) Glutamate = “fast” neurotransmitter. Also may release ATP and maybe acetylcholine (this last one is from Mike G.). c) Also release neuropeptides aka co‐neurotransmitters : substance P (SP), calcitonin gene‐related peptide, cholecystokinin (CCK), somatostatin, galanin, neuropeptide Y (NPY), vasoacitive intestinal peptide (VIP) and bombesin. d) The “fast” neurotransmitters are released via small clear vesicles (as defined by electron microscopic studies). Peptides are contained in larger dense core vesicles. More Neurochemistry 1) One of the most commonly used classifications for C‐fiber nociceptors is “peptidergic” vs. “non‐peptidergic”. 2) The peptides in “peptidergic” are primarily SP and CGRP. Non‐ peptidergic neurons = those that express gangliosides that bind an isolectin from an African plant Griffonia (bandeiraeia) simplicifolia (IB4). They also express the GPCR MrgD (Mas‐ related gene) 3) In general, these two different classes of neurons have different central and peripheral projections, growth factor dependencies and differentially express other nociceptor‐ specific proteins. Nociceptor Neurochemistry (cont) * Human sensory neurons do not stain for IB4 ‐ the gene responsible for adding this sugar residue is a pseudogene. Physiology of nociceptors a) Nociceptors are best identified based on their function, i.e., their ability to detect noxious stimuli. b) This is the concept called ADEQUATE STIMULUS and goes back to Sherrington (1906). Physiology of nociceptors (cont) Nociceptor Terminology: i) C‐fibers that respond to noxious mechanical and thermal stimuli = C‐mechanoheat (CMH) or C‐mechanocold (CMC) (aka polymodal nociceptors). But there are also CM and CH and probably CC (i.e. cells responding to one modality). ii) A‐fibers may be named in a similar manner (e.g. AMH or AMC). iii) Another terminology you will see is Mechanical Sensitive Afferent (MSA) and Mechanically Insensitive Afferents (MIA). iv) You will also see A‐fiber nociceptors referred to as High Threshold Mechanoreceptors (AHTM or HTMR; note the H is for high threshold not heat). Examples of cutaneous nociceptor coding (RIGHT). Intracellular recording from a HTMR during mechanical stimulation of increasing intensity (BOTTOM) Intracellular recordings from 4 heat sensitive C‐fiber polymodal nociceptors. These neurons show coding for the heat ramp and some exhibit an “off‐like” response at the end of the ramp. Example of visceral nociceptor coding a) Single fiber response of visceral nociceptors during colorectal distension. b) Firing frequency increases with stimulus intensity. c) There appears to be two type of visceral nociceptors – low threshold (77% in rat colon) and high thresholds (23% in rat colon). d) Both are usually chemically sensitive and can be sensitized by insults. e) Are low threshold fibers are still nociceptors?. From Sengupta and Gebhart, J. Neurophys 1994, 71:2046 A modest proposal All afferents are nociceptors. No afferents are nociceptors. X% of afferents are nociceptors all of the time and X% of afferents are never nociceptors. The rest are inbetweeners* *Paraphrasing the Kinsey report 1948 Take home message from Part I 1) Nociceptors probably evolved from cells/neurons whose job was not nociception. 1) Nociceptors may not always be nociceptors 2) The answers you get depends on how you ask the question. 3) When possible, be very specific about the cells you’re talking about – “capsaicin‐sensitive bladder C‐fiber”. 4) Use the term “nociceptor” at your own risk. The search for signal transduction molecules The search for signal transduction molecules has taken a number of routes: 1) Search knockouts in lower organisms (e.g. C. elegans) for behavioral deficits that indicate loss of transduction mechanisms (e.g. loss of mechanical sensitivity identified members of ENaC family that may contribute to mechanical sensitivity). 2) Use ligands known to produce particular sensations to identify genes responsible for that sensation ‐ Used to identify thermally sensitive channels. 3) For molecular biologist the holy grail would be to find a single gene that could be used to identify nociceptors – Is this realistic??? The cloning and characterization of TRPV1 (VR1) Caterina et 2000 J.B. Davis et al., 2000 Cloning of TRPV1 a) b) c) d) Caterina et al used a cDNA library from sensory neurons, transfected HEK cells with different genes and tested them for their response to capsaicin Once TRPV1 identified he could test cells for heat response. Final test was to make knockout mice. TRPV1 responds to noxious heat, protons and endogenous product of Caterina et al Nature (1997) 389:816 TRPA1 TRPV1 H+ A A A N C A A A A A A A A A A A A A A A A N C •Both TRPV1 and TRPA1 contain 7 transmembrane domains •Both binds specific chemical ligands •Both contain ankyrin domains ‐ interact with many other proteins Once you have one gene, finding others is easy From Tominaga and Caterina 2003 In heterologous systems each TRP channel has different thermal properties. Does this mean that this is how they function in afferents? From Tominaga and Caterina 2003 How do we determine what a gene does? 1)Old school: Knock it out completely (from conception) Mario Capechhi, Martin Evans and Oliver Smithies (1989) Nobel Prize 2007 What is the role of TRPV1 in heat detection? The majority (ca. 70%) of cutaneous afferents in TRPV1 knockout mice have normal heat response Heat threshold and firing frequency is normal in TRPV1 ‐/‐ mice ……..for CMH neurons My confession – there are heat sensitive afferents that are missing in TRPV1‐/‐ mice. Why did we miss these? Approximately 10% of cutaneous afferents are CH fibers TRPV1‐/‐ mice lack CH fibers WT TRPV1‐/‐ % labeled TRPV1 ‐ immunoreactivity is expressed by a higher percentage of colonic and femoral afferents than saphenous afferents 100 90 80 70 60 50 40 30 20 10 TRPV1 CGRP ## ** * # ** ## * ** Colon Femoral Saph (CTB) (WGA) (WGA) Saph (IB4) N52 * # # Colon Femoral Saph (CTB) (WGA) (WGA) Saph (IB4) IB4 # Colon Femoral Saph (CTB) (WGA) (WGA) * Saph (IB4) Colon Femoral Saph (CTB) (WGA) (WGA) ND Saph (IB4) TRPV1 is more commonly and robustly expressed in muscle and visceral afferents A Skin 31 ± 4% * Muscle 67 ± 8% Colon 74 ± 4% 0.3 ΔF 20 s * * cap B Skin 19 ± 3%* Muscle 53 ± 6% Colon 70 ± 4% 0.2 ΔF 20 s * mo * If cutaneous heat responses are ca. normal in TRPV1‐/‐ mice, and TRPV1 is more often expressed in deep tissue afferents, what is it role? wt ‐/‐ wt ‐/‐ In the absence of TRPV1, inflammation does not produce hypersensitivity; i.e. TRPV1‐expressing neurons may be required for development of persistent pain. For TRPV1, traditional knockout mice were an imperfect tool. What’s our alternative? ADULT ABLATIONS DEMONSTRATES IMPORTANCE OF TRPV1‐ EXPRESSING CELLS Resiniferatoxin (RTX) TRPV1 super agonist ‐ binds and produces Ca++ cytotoxicity Tested in dogs in neoplasm and/or arthritis Applied single dose intrathecally Maintained efficacy even as cancer progressed. Karai et al. 2004; J. Clin Invest. Does the previous experiment prove that TRPV1 is necessary for cancer and arthritis pain? What about ablating other populations of putative nociceptors? (Shields et al 2010) Ablated TRPV1‐expressing spinal terminals with i.t. capsaicin and IB4/MrgD neurons with (i.p.) diptheria toxin TRPV1 ablation decreases response to low dose (0.5%) formalin Mustard oil TRPV1 ablation does not affect high dose (2%) formalin hyperalgesia Ablation of MrgD neurons does not affect low dose formalin Double ablation doesn’t do it either for high dose formalin. Other important data regarding TRPV1 1)TRPV1‐/‐ mice have increased mechanical thresholds for visceral afferents (Jones et al 2005). 2)TRPV1 is often coexpressed with TRPA1 (why would cold and hot receptors be expressed in the same cells?). 3)P2Y2 (a GPCR, see below) is coexpressed with TRPV1 and P2Y2‐/‐ mice have a more dramatic thermal phenotype than TRPV1‐/‐ mice (Malin et al 2008). What is the role of TRPV1? 1) Inflammatory hyperalgesia 2) “Nociceptive tone” 3) Context dependent 4) Allow cations to flow into cell in response to multiple stimuli – Safe bet. New transducer molecules for 2010 Coste et al 2010 Science 330:55 1) Patapoutian lab poked N2 A cells with patch electrode looking for “mechanically activated currents 2) Did gene chips to determine what proteins were found in cells and then used siRNA to knock down one gene at a time to determine if MA currents were diminished. 3) Found 2 “Piezo” proteins – highly conserved in vertebrate. Related proteins found in protozoa. 4) Piezo 2 expressed in multiple types of DRG neurons and siRNA to piezo 2 can decrease MA Nociceptors express many other membrane‐ bound proteins that regulate function 1) Ligand‐gated receptors a) Ionotropic (channels) b) Metabotropic 2) Voltage gated channels ligand‐gated ionotropic receptors a) ligand‐gated = binding of ligand for activation – molecules of many sizes can work. b) IONOTROPIC receptors include a pore forming region and allow ions to flow, directly affecting the membrane potential. Some of these receptors bind classical neurotransmitters (e.g. glutamate, GABA). Others respond to exogenous compounds. Metabotropic Receptors 1) Metabotropic receptor = “a receptor where activation leads to some change in metabolic processes within the cell, rather than opening or closing of an ion channel (as with the ionotropic receptor)”. 2) G‐protein coupled receptors (GPCR) are very potent and important in sensitizaiton. 3) There are several hundred GPCRs in the human genome. Rhodopsin is the oldest evolutionarily, and was the first GPCR to have its structure determined by X‐ray crystallography (Palczewski et al 2000). 4) GPCRs have characteristic conformation including 7 transmembrane domains and a binding site located in the extracellular or transmembrane domain or even a site that overlaps both (as is true for opiate receptors) MOR = µ Opioid Recptor VDCC = voltage dependent calcium channel GIRK = G-protein-regulatetd inward rectifying potassium channel MOR-Gi/o VDCC Gi βγ βγ (- ) (+) TRPV1 Increased Pain AC = adenylate cyclase PKA = protein kinase A PKC = protein kinase C PLC = phospholipase C (+) AC Nucleus CREB ER PKC DAG IP3 PLC Ca++ βγ Gq βγ NaV1.8 PKA βγ (- ) Decreased Pain Gs (+) GIRK P2Y2-Gq (ATP) EP2-GS (PGE2 prostaglandin) Metabotropic Receptors (cont) a) Many metabotropic receptors have matching ionotropic receptor partners and may be expressed in the same cell. b) These receptors can be expressed peripherally and/or centrally. Collaboration between ionotropic and metabotropic ATP receptors a) b) c) d) Single cell recordings from sensory neurons exposed to ATP. ATP binds P2X3 which is an ionotropic receptor. But ATP also binds P2Y2 which is a metabotrpic receptor. αβme‐ATP is selective for P2X3, but only produces initial burst. Test same cell with UTP which is selective of P2Y2 and get delay followed by prolonged bursting. e) Thus, the complex response in this cell to ATP is a collaboration between two different types of ATP receptors, one ionotropic and on metabotropic. Growth factor receptors are also metabotropic receptors a) Growth factor receptors are required for sensory neuron survival during development. b) The great majority of nociceptors start out life being trkA‐positive and requiring NGF for survival. c) Late in development, ca. 1/2 of these down‐ regulate trkA and being expressing ret plus one of the GFRα receptors. In the adult, nociceptors continue to respond to NGF, artemin, neurturin and GDNF GDNF Family GDNF Neurturin GFRα1 Neurotrophin Family Artemin GFRα2 Ret GFRα3 Ret NGF p75 NT‐3 p75 trkA BDNF/ NT‐4 p75 trkC trkB • Nociceptors express trkA and/or ret, plus GFRα 1‐3 • All of these growth factors are expressed in the targets of sensory neurons, both somatic and visceral. • More recent work (Valdes‐Sanchez, Devel Bio 2010 339:465) indictates trkB required for survival of putative nocicpetors Voltage gated channels Membrane Channel properties affected Ion role Name Passive (prior to AP) K Active at resting membrane potential, contrib. to resting input resistance. Potential transducer of stretch TREK-1 (a tandem of P domains in a weak inward rectifier potassium channel (TWIK) Mechanosensitive TREK-2 Mechanosensitive TRAAK Active (during AP) Two pore potassium channel Nav 1.9 Na Active at rest Regulate neuronal excitability TTX resistant NaV 1.9 Voltage gated sodium channels Na Spike initiation TTX resistant AP conduction AP generation (1.7) TTX sensitive High in development TTX insensitive NaV 1.8 High in development TTX sensitive Nav 1.3 TTX sensitive Nav 1.1 Nav 1.2 Nav 1.6 Nav 1.7 Nav 1.5 Voltage gated channels (cont) Membrane properties affected AP downstroke AHP See Mikes Lecture Channel Ion role Name Voltage gated potassium channels K Delayed rectifier Large conductance Ca modulated K channels T-type voltage gated Ca channel Hyperpolarizationactivated cyclic nucleotide –gated channels (HCN) Voltage gated Ca channels K Contribute to AHP Iaht (Kv3.4) Iki (Kv1.2) Ias (Kv1.1) BK or Maxi-K Regulate AHP HCN Ca Nonselective cation Ca L (Cav1) Neurotransmission Target for neuropathic pain Target of Omega & conotoxin opiates Neurotransmission Migraine Neurotransmission N (Cav2) P(Cav2) R(Cav2) T (Cav3) New things specific to nociceptors • Epigenetic factors – basis of chronic pain? Epigenetic (cont) • DNA – methylation • Histones – acetylation, methylation, ubiquitylation, phosphorylation, sumoylation Epigenetic Gene Silencing Underlies C‐Fiber Dysfunctions in Neuropathic Pain Uchida et al J. Neurosci. 2010 30:4806 NRSF – neuron‐restrictive silencer factor: recruits histone deacetylase to produce “repressive chromatin environment” Binds to genes that express NRSE – neuron restrictive silencer element MOR has NRSE and Nav1.8 has forward and reverse NRSEs. • NRSF is increased following partial sciatic ligation • NRSF is correlated with increase acetylation of histone 4 • NRSF protein is increased in DRG and it appears to be neuronal • Antisense NRSF can block injury induce down regulation of MOR and Nav 1.8. • Similar effects on TRPM8 and TRPA1 • Antisense NRSF restores some morphine sensitivity Efferent function for sensory neurons a) That nociceptors could have efferent properties was first proposed by Stricker in 1876 . b) Bayliss (1901, 1902) found that stimulation of dorsal root produced vasodilation that we now know to be due to release of neuropeptides like CGRP from sensory endings. c) Subsequent studies showed the sensory neurons were responsible for “neurogenic inflammation” which has two components: vasodilation and plasma extravasation. Vasodilation produces the flare associated with capsaicin or mustard oil application to the skin whereas plasma extravasation produces the wheal and edema at the site of the injury. Four potential mechanisms for neuropeptide release from nociceptors 1) AP spread to axonal branches 2) Axonal reflexes 4) Spontaneous activity 3) Dorsal Root Reflex