* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Vestibular Signals in the Parasolitary Nucleus
Apical dendrite wikipedia , lookup
Neural modeling fields wikipedia , lookup
Convolutional neural network wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Neuroplasticity wikipedia , lookup
Types of artificial neural networks wikipedia , lookup
Environmental enrichment wikipedia , lookup
Haemodynamic response wikipedia , lookup
Synaptogenesis wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Electrophysiology wikipedia , lookup
Neuroscience in space wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Neurotransmitter wikipedia , lookup
Axon guidance wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Transcranial direct-current stimulation wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Single-unit recording wikipedia , lookup
Multielectrode array wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Mirror neuron wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Biological neuron model wikipedia , lookup
Neural oscillation wikipedia , lookup
Hypothalamus wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Neurostimulation wikipedia , lookup
Neural coding wikipedia , lookup
Metastability in the brain wikipedia , lookup
Evoked potential wikipedia , lookup
Central pattern generator wikipedia , lookup
Neuroanatomy wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Circumventricular organs wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Nervous system network models wikipedia , lookup
Optogenetics wikipedia , lookup
Channelrhodopsin wikipedia , lookup
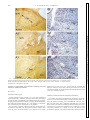
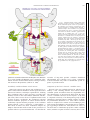
Vestibular Signals in the Parasolitary Nucleus N. H. BARMACK AND V. YAKHNITSA Neurological Sciences Institute, Oregon Health Sciences University, Portland, Oregon 97201 INTRODUCTION The anatomical entity termed the Parasolitary nucleus (Psol) derives its name primarily because of its proximity to the nucleus solitarius and tractus solitarius. It is located dorsal and lateral to these structures. It extends rostrocaudally for about 2 mm and in its most rostral extent lies wedged between the medial and descending vestibular nuclei (MVN and DVN). Rather than being directly a part of a baroceptive regulation, the Psol is really the most caudal subdivision of the vestibular complex. The Psol consists of a compact cluster of 2,300 smalldiameter neurons (5–7 m diam) extending from the nucleus solitarius to the surface of the fourth ventricle (Barmack et al. 1998). In the rabbit, the Psol is identifiable using either cytoarchitectonic or immunohistochemical criteria (Fig. 1B). All The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. Psol neurons are labeled by an antiserum to glutamic acid decarboxylase (GAD; Fig. 1A), the synthetic enzyme for the neurotransmitter gamma amino butyric acid (GABA) (Barmack et al. 1998). Vestibular primary afferents project to Psol, demonstrated by the distribution of neuronal degeneration following lesion of the vestibular nerve (Korte 1979). This projection is also revealed by the orthograde transport of the C-fragment of tetanus toxin (TTC) into vestibular primary afferents terminals in the Psol following injection of TTC into the membranous labyrinth through the oval window (Barmack, unpublished observations). The Psol receives secondary vestibular afferent projections from neurons in the DVN and MVN (Ruggiero et al. 1996) and a bilateral descending projection from the fastigial nuclei that is shared with other vestibular nuclei (Walberg et al. 1962a,b). The Psol also receives projections from cerebellar Purkinje cells located in the ipsilateral uvulanodulus (N. H. Barmack, Z.-Y. Qian, and J. Yoshimura, unpublished data). Unlike the nucleus solitarius, the Psol receives no sensory fibers from cranial nerves VII, IX, and X (Allen 1923; Altschuler et al. 1989). Evidence from both orthograde and retrograde tracer studies indicates that the Psol is the source of the GABAergic projections to the -nucleus and dorsomedial cell column (dmcc) of the inferior olive and the nucleus reticularis gigantocellularis (NRGc) (Barmack et al. 1998; Fagerson and Barmack 1995; Kaufman et al. 1996) (Fig. 1). Singleneuron recordings from the -nucleus, dmcc, and NRGc indicate that neurons in these nuclei respond to vestibular stimulation with increases during contralateral static and dynamic roll-tilt about the longitudinal axis (Barmack 1996; Barmack et al. 1993a; Fagerson and Barmack 1995). The -nucleus and dmcc send climbing fiber projections to the contralateral uvula-nodulus where vestibular-related climbing fiber responses are mapped onto sagittal strips (Barmack and Shojaku 1995; Fushiki and Barmack 1997). These strips encode ipsilateral static and dynamic roll-tilt. In the uvulanodulus, as in the -nucleus and dmcc, no modulation of activity is evoked by vestibular stimulation in the plane of the horizontal semicircular canals. Since the anatomical evidence indicates that the Psol is involved in the processing of vestibular information, in this experiment we have examined how Psol neurons encode natural vestibular stimulation. We have sought answers to three questions: are Psol neurons modulated by physiological vestibular stimulation, do signals from all vestibular end organs drive Psol neurons, and do Psol neurons receive convergent optokinetic signals? We answered these questions by using the 0022-3077/00 $5.00 Copyright © 2000 The American Physiological Society 3559 Downloaded from http://jn.physiology.org/ by 10.220.32.246 on June 14, 2017 Barmack, N. H. and V. Yakhnitsa. Vestibular signals in the parasolitary nucleus. J Neurophysiol 83: 3559 –3569, 2000. Vestibular primary afferents project to secondary vestibular neurons located in the vestibular complex. Vestibular primary afferents also project to the uvula-nodulus of the cerebellum where they terminate on granule cells. In this report we describe the physiological properties of neurons in a “new” vestibular nucleus, the parasolitary nucleus (Psol). This nucleus consists of 2,300 GABAergic neurons that project onto the ipsilateral inferior olive (-nucleus and dorsomedial cell column) as well as the nucleus reticularis gigantocellularis. These olivary neurons are the exclusive source of vestibularly modulated climbing fiber inputs to the cerebellum. We recorded the activity of Psol neurons during natural vestibular stimulation in anesthetized rabbits. The rabbits were placed in a three-axis rate table at the center of a large sphere, permitting vestibular and optokinetic stimulation. We recorded from 74 neurons in the Psol and from 23 neurons in the regions bordering Psol. The activity of 72/74 Psol neurons and 4/23 non-Psol neurons was modulated by vestibular stimulation in either the pitch or roll planes but not the horizontal plane. Psol neurons responded in phase with ipsilateral side-down head position or velocity during sinusoidal stimulation. Approximately 80% of the recorded Psol neurons responded to static roll-tilt. The optimal response planes of evoked vestibular responses were inferred from measurement of null planes. Optimal response planes usually were aligned with the anatomical orientation of one of the two ipsilateral vertical semicircular canals. The frequency dependence of null plane measurements indicated a convergence of vestibular information from otoliths and semicircular canals. None of the recorded neurons evinced optokinetic sensitivity. These results are consistent with the view that Psol neurons provide the vestibular signals to the inferior olive that eventually reached the cerebellum in the form of modulated climbing fiber discharges. These signals provide information about spatial orientation about the longitudinal axis. 3560 N. H. BARMACK AND V. YAKHNITSA technique of extracellular microelectrode recording from Psol neurons in anesthetized rabbits. METHODS Anesthesia and surgery Twenty pigmented rabbits (weight 0.8 –1.7 kg) were anesthetized intravenously with ␣-chloralose (50 mg/kg) and urethan (500 mg/kg) or intramuscularly with ketamine hydrochloride (50 mg/kg), xylazine (6 mg/kg), and acepromazine maleate (1.2 mg/kg). Rectal temperature was monitored and maintained at 37°C. The adequacy of anesthesia was evaluated using the corneal reflex as an indicator. In a preparatory operation, a dental acrylic plug was formed to the calvarium of each rabbit. This plug held two inverted stainless steel screws (8 –32) to the dorsal surface of the calvarium. Five smaller stainless steel screws (2–56) were screwed into the calvarium and helped to anchor the acrylic plug. The larger inverted screws mated with a metal rod that was used to maintain the head rigidly fixed in the center of a vestibular rate table. Glutamic acid decarboxylase immunocytochemistry Two rabbits were deeply anesthetized (as described in the preceding text) and perfused transcardially with physiological saline followed by a fixative consisting of 4% formaldehyde, 0.2% zinc salicylate, and 0.9% NaCl, pH 6.5 (Mugnaini and Dahl 1983). The brain stems rostral to the obex and caudal to the dorsal cochlear nuclei were removed and cryo-protected in graded sucrose in saline prior to being frozen and sectioned on a cryostat at 30 m. The sections were processed for glutamic acid decarboxylase (GAD) immunocytochem- Downloaded from http://jn.physiology.org/ by 10.220.32.246 on June 14, 2017 FIG. 1. Parasolitary nucleus (Psol) topography in the rabbit. A, 1–3: transverse sections immunolabeled with antiserum to glutamic acid decarboxylase (GAD). Sections are spaced ⬃500 m apart, from rostral (A1 to caudal A3). B, 1–3: histology of Psol neurons identified with Klüver-Barrera stain. MVN, medial vestibular nucleus; Nsol, nucleus solitarius; sol, tractus solitarius. Calibration bar in B3 applies to all panels. PARASOLITARY VESTIBULAR PHYSIOLOGY 3561 istry as previously described using a double-peroxidase–anti-peroxidase protocol (Barmack et al. 1998). Vestibular stimulation The head of the rabbit was held rigidly in the center of rotation of a three-axis vestibular rate table with the plane of the horizontal semicircular canals maintained in the earth horizontal plane. The body of the rabbit was encased in foam rubber and fixed with elastic straps to a plastic tube aligned with the longitudinal axis of the rate table. The rate table was sinusoidally oscillated about its vertical axis (yaw), about its longitudinal axis (roll), or about its interaural axis (pitch) (⫾10°, 0.005– 0.800 Hz). During vestibular stimulation the vision of the rabbit was occluded by hemispherical ping pong balls. Static vestibular sensitivity Static vestibular stimulation was used to test for the otolithic origin of vestibularly evoked discharges. The rabbit was tilted 5–10° about the longitudinal axis. After an adaptation period of 20 –30 s, the average discharge frequency was measured for the next 20 –30 s. The rabbit was then tilted in the opposite direction. A difference in mean discharge frequency of 20%, evoked for roll tilts in the two opposite directions, indicated sensitivity to linear acceleration. Based on the predominant mediolateral polarization vector of hair cells within the utricular maculae, as opposed to the predominant ventral-dorsal polarization vectors for hair cells within the saccular maculae (Fernandez and Goldberg 1976), we presume that a static roll stimulus evoked activity originating primarily from utricular hair cells. Vestibular null plane measurement A “null technique” was used to characterize the peripheral origins of the vestibular signals that modulated the activity of Psol neurons. While the rabbit was rotated about the longitudinal axis of the rate table, the angle of the rabbit’s head was changed about the vertical axis until a minimum in stimulus-modulated neuronal activity was detected (null plane). On either side of this null plane, the phase of modulated activity was shifted with respect to the vestibular forcing function by 180°. For each tested neuron, the null plane characterized the polarization vector of the hair cells of particular end organs that contributed to the modulated response. The “optimal plane,” the plane of maximal neuronal modulation was assumed to be orthogonal to the null plane (Fushiki and Barmack 1997). Downloaded from http://jn.physiology.org/ by 10.220.32.246 on June 14, 2017 FIG. 2. Activity of a Psol neuron evoked by sinusoidal roll vestibular stimulation. A: vestibular stimulation about the longitudinal axis evoked maximal modulation of neuronal activity when the left posterior semicircular canal was orthogonal to the axis of rotation. The head of the rabbit was oriented 45° clockwise (CW) with respect to the longitudinal axis. Neuronal activity increased during rotation onto the left side and decreased during rotation onto the right side. The sign wave at the bottom of the figure indicates rotation about the longitudinal axis. An upward deflection indicates rotation onto the left side. B: when the head of the rabbit was oriented 39° counterclockwise (CCW) with respect to the longitudinal axis, the activity of the Psol neuron was not modulated by sinusoidal roll. In this “null plane,” the left posterior semicircular canal was colinear to the axis of rotation. C: when the head of the rabbit was oriented 48° CCW with respect to the longitudinal axis (about 9° past the null plane), modulation of neuronal activity again appeared but with a phase shift of 180° with respect to the sinusoidal stimulus. The location of the recorded neuron within the Psol is illustrated in D. Amb, nucleus ambiguus; Cu, cuneate nucleus; dmcc, dorsomedial cell column of the inferior olive; icp, inferior cerebellar peduncle; LPC, RAC, left posterior and right anterior semicircular canals; DVN, descending vestibular nuclei; NPH, nucleus prepositus hypoglossi; NRGc, nucleus reticularis gigantocellularis; SpV, spinal trigeminal nucleus. 3562 N. H. BARMACK AND V. YAKHNITSA Optokinetic stimulation The rate table was located at the center of a large sphere (1.45 m diam). A 120° segment was cut out of the sphere to allow convenient access to the animal. Nonetheless it was possible to orient the rabbit so that the visual field of a single eye was completely encompassed by the interior surface of the sphere. A perforated aluminum globe (4 cm diam), with a 50-W tungsten filament microscope bulb on the inside, was positioned just over the head of the rabbit. The globe was mounted on a small pen motor. Movement of this globe was controlled by voltage ramps to the pen motor. The globe-pen motor could be aligned with any axis to create movement about that axis. The optokinetic sensitivity of Psol neurons was tested for stimulation about the vertical, longitudinal, and oblique axes. Microelectrode recording The Psol was approached by reflecting the muscles overlying the cisterna magna and enlarging the dorsal aspect of the foramen magnum. The dura mater was carefully removed exposing the dorsal surface of the brain stem and folia 9c of the cerebellum. The meningeal attachments of the cerebellum were carefully cut, allowing the cerebellum to retract 1–2 mm rostrally. A hydraulic microdrive, attached to the head-restraint bar, advanced tungsten microelectrodes toward a region of the dorsal brain stem just caudal to the medial vestibular nucleus about 1.8 mm lateral to the midline. The micro- electrodes had an extended taper so that the diameter of the final 4 mm was ⬍50 m. The tip impedance was ⬃4 M⍀. Action potentials were discriminated with a window-slope discriminator-Schmitt trigger. Discriminated action potentials were analyzed by computer using Spike 2 software (Cambridge Electronic Design). Evoked single-unit activity was displayed on-line as a peristimulus histogram. Peristimulus histograms were constructed using different numbers of stimulus cycles at different stimulus frequencies. During data acquisition, each stimulus cycle was divided into 180 bins. Interspike intervals for spike occurrences were stored in these bins. The reciprocal of these interspike intervals, spike frequency, was averaged for the number of spike occurrences in each bin. Histological verification of recording sites The location of each neuron from which recordings were obtained was marked electrolytically (⫺8 A, 30 s). At the conclusion of the experiment each rabbit was deeply anesthetized and perfused transcardially with 0.9% saline, followed by 10% paraformaldehyde. The brain was removed and cryoprotected with 10, 20, and 30% sucrose in 0.1 M PBS, pH 7.2. The brain stem was blocked sagittally, mounted onto cork with OCT compound, and frozen in isopentane cooled with dry ice. Sagittal frozen sections (35 m) were cut and collected in cold 0.1 M PBS and mounted serially. The location of each recorded neuron was reconstructed from the locations of the marking lesions. These locations were analyzed in transverse histological sections and Downloaded from http://jn.physiology.org/ by 10.220.32.246 on June 14, 2017 FIG. 3. Responses of a Psol neuron to static roll. Step-roll stimulation was used to characterize the otolithic sensitivity of Psol neurons. A: this neuron, recorded from the left Psol increased its rate of discharge when the rabbit was rolled onto its left side. The step response at the bottom of the figure indicates step-roll about the longitudinal axis. An upward deflection indicates step-roll onto the left side. B: cartoon indicates the optimal response plane for the Psol neuron whose responses are shown in A. C: the peristimulus histogram for this neuron illustrates 2 complete cycles of vestibular step roll-tilt. D: this neuron was located in the Psol ⬃500 m rostral to its caudal pole. The rectangle demarcates the area of the photomicrograph in E. 2, microlesion that marked the location of the neuron. X, dorsomotor nucleus of the vagus. PARASOLITARY VESTIBULAR PHYSIOLOGY 3563 two discharge rates reflects the stimulus-driven activity of many otherwise quiescent Psol neurons. We measured the null plane of 47/74 Psol neurons from which we recorded and inferred the optimal response planes from these null planes. During sinusoidal vestibular stimulation about the longitudinal axis, the orientation of the head of the rabbit was systematically changed until a minimum in the evoked modulation of Psol activity was reached (Fig. 2B). On either side of the null plane the modulation of activity was phase shifted by 180° (Fig. 2, A and C). The optimal plane for the neuron illustrated in Fig. 2 aligned with the ipsilateral posterior and contralateral anterior semicircular canals (Fig. 2A). Absence of Psol responses to horizontal vestibular stimulation Absence of optokinetic modulation of Psol neuronal activity The activity of none of the neurons localized to Psol could be modulated by optokinetic stimulation. The absence of a modulated optokinetic response was not dependent on the particular alignment of the optokinetic stimulation. There was no modulation of Psol activity when the optokinetic stimulation axis was coaxial as well as orthogonal to the optimal axis of vestibular stimulation. FIG. 4. Influence of stimulus frequency on a cell with static vestibular sensitivity. A: a peristimulus histogram for a neuron recorded in the left Psol during static roll-tilt stimulation indicates sensitivity to static tilt. B: the figurine indicates that the optimal plane for this neuron was 36° CCW to the longitudinal axis. C: 1, location of the neuron in the rostral Psol. D: peristimulus histograms were constructed of the response of this Psol neuron tested at different sinusoidal stimulus frequencies, all delivered with the head maintained in the optimal plane. Stimulus frequencies are indicated for each panel. The response lead head position by ⬃40° at a stimulus frequencies of 0.02– 0.40 Hz. At the highest frequency examined, the response lagged head position by 20 –30°. Numbers in each panel refer to the number of stimulus cycles that were used to obtain the peristimulus histograms. XII, hypoglossal nucleus. Static roll responses of Psol neurons In addition to testing each suspected Psol neuron with vestibular sinusoids, we examined the static responses of Psol then transferred onto one of four templates spaced about 500 m apart representing the rostrocaudal extent of the Psol. RESULTS Activity evoked in Psol neurons by vestibular stimulation: optimal and null response planes We recorded from a total of 97 neurons, 74 of which were localized to the Psol. Sinusoidal oscillation of the rabbit about its longitudinal axis modulated the activity of Psol neurons (Fig. 2). When the rabbit was rotated onto the side that was ipsilateral to the recorded Psol neuron, an increase in discharge rate was evoked. When the rabbit was rolled onto the side contralateral to the recorded Psol neuron, there was a decrease in discharge rate. Rotation of the rabbit about its vertical axis (yaw stimulation) failed to modulate Psol activity. The average discharge rate during sinusoidal stimulation of 74 Psol neurons was 13.3 ⫾ 9.5 (SD) imp/s. The average resting discharge rate for a smaller sample (n ⫽ 21) of Psol neurons was 9.6 ⫾ 10.5 imp/s. The discrepancy between these FIG. 5. Phase of responses in Psol neurons. A: in 47 Psol neurons, the phase of the vestibularly evoked responses relative to the sinusoidal forcing function were measured at a stimulus frequency of 0.3– 0.4 Hz while the rabbit’s head was maintained in an optimal plane. B: in 13 Psol neurons, a complete series of phase measurements were made. The responses are consistent with convergent inputs from both the vertical semicircular canals and utricular otoliths. Downloaded from http://jn.physiology.org/ by 10.220.32.246 on June 14, 2017 Only one neuron responded optimally to horizontal vestibular stimulation. This neuron was localized to the border between the Psol and the MVN. 3564 N. H. BARMACK AND V. YAKHNITSA neurons using static roll-tilt (see METHODS). More than 80% of recorded Psol neurons had a positive static roll-tilt response. A subset of these neurons was also tested with periodic vestibular step stimulation. The optimal response plane for evoking activity from these neurons was based on previous testing with vestibular sinusoids. An example of a neuron that tested positive for static roll is illustrated in Fig. 3. This neuron responded when the rabbit was stepped onto its left side (Fig. 3A). A peristimulus histogram illustrating two cycles of step roll-tilt indicates that the response did not adapt during the brief (10 s) step (Fig. 3C). This neuron was localized to Psol at a level that was 1,000 m rostral to the caudal pole of the Psol (Fig. 3, D and E). Another Psol neuron, with a positive static roll-tilt test, was examined with a series of sinusoidal frequencies (Fig. 4). The evoked discharge of this neuron lead head position by ⬃40° at a stimulus frequencies of 0.02– 0.10 Hz (Fig. 4D). However, at the highest two frequencies, the gain of the response was reduced and the phase of the response lagged head position by 20 –30°. This neuron was localized to the most rostral part of the Psol, 2,000 m rostral to the caudal pole of the Psol (Fig. 4C). The phase of 47 Psol neurons, each with a static roll response, was compared using vestibular sinusoids at 0.30 – 0.40 Hz (Fig. 5A). The mean phase lead of this population was 39.5 ⫾ 5.1°. Only four neurons had a phase lag at the frequencies tested. A full range of vestibular sinusoids was used to examine the phase of the evoked discharge in a subset of 13/47 Psol neurons (Fig. 5B). The phase lead with respect to head position decreased by ⬃40° as the frequency of stimulation was increased from 0.10 to 0.80 Hz. Frequency dependence of null plane analysis The foregoing data suggested that Psol neurons receive convergent vestibular information. At least some of this convergence could be attributed to signals arising from the utricular otoliths and vertical semicircular canals. If this were true, then it should be possible to obtain a frequency-dependent shift in the null plane of a neuron. We measured the null plane at two different frequencies (0.02 and 0.30 Hz) for the neuron illustrated in Fig. 6. This neuron had a positive response to static tilt, demonstrating that it received otolith information. In such neurons, it was not feasible to conclude that the response at higher frequencies was driven by either otoliths, semicircular canals or combinations of these inputs. The null plane for the higher frequency corresponded to a null plane of 45° counterclockwise (CCW; Fig. 6B). The null plane at the lower frequency corresponded to a head orientation of 17° CCW Downloaded from http://jn.physiology.org/ by 10.220.32.246 on June 14, 2017 FIG. 6. Influence of stimulus frequency on measurement of null plane. The vestibular null plane was measured in a left Psol neuron at 2 different stimulus frequencies. Peristimulus histograms (B and C) were obtained with the head maintained in the positions shown in A and during sinusoidal vestibular stimulation with a 0.30 and 0.02 Hz. The null plane was frequency dependent. The null plane at 0.30 Hz (B) aligned with a head orientation of 45° CCW with respect to the longitudinal axis. The null plane in C aligned with a head orientation of 17° CCW with respect to the longitudinal axis. The numbers in each panel refer to the number of stimulus cycles that were used to obtain the peristimulus histograms. PARASOLITARY VESTIBULAR PHYSIOLOGY 3565 vestibular stimulation about the longitudinal axis. Twenty-six neurons were localized within the Psol and had optimal response planes consistent with stimulation of the ipsilateral anterior semicircular canal (E). These neurons were concentrated in the most rostral region of Psol (Fig. 8D). Twenty-nine neurons had optimal response planes consistent with stimulation of the ipsilateral posterior semicircular canal (F). A topographic concentration of these neurons within the Psol was not evident. Finally, four neurons were found with a Type 3 response (䡲). These neurons responded to sinusoidal rotation onto the left and right sides. Vestibularly evoked oscillations in Psol neurons In most Psol neurons vestibularly evoked discharges were stationary. They were repeatedly evoked, for tens of minutes, during sinusoidal rotation of the rabbit about the longitudinal Downloaded from http://jn.physiology.org/ by 10.220.32.246 on June 14, 2017 FIG. 7. Distribution of optimal response planes for Psol neurons. Null planes were measured in 47 Psol neurons at sinusoidal stimulus frequencies of 0.20 – 0.40 Hz. The optimal response planes of these neurons clustered about the anatomical orientation of the ipsilateral anterior and posterior semicircular canals. The numbers adjacent to each bracketed set of optimal response planes indicate the mean for that cluster and the number, n, of Psol neurons included in the average. (Fig. 6C). These data suggest convergence of at least two different vestibular primary afferents at the level of the Psol neuron. Distribution of optimal response planes for Psol neurons We measured the null planes in 47 Psol neurons at stimulus frequencies of 0.20 – 0.40 Hz. The optimal response planes of most of these neurons aligned with the anatomical orientation of either the ipsilateral anterior or posterior semicircular canals (Fig. 7). Topographic distribution of optimal response planes mapped onto the Psol For each neuron from which we recorded, a microlesion was made to mark its location. The location of these marking lesions was reconstructed from transverse histological sections through the Psol and plotted onto standard transverse schematics spaced 500 m apart (Fig. 8). The locations of 97 recorded neurons are represented. Of these neurons, 21 were unresponsive to vestibular stimulation about the longitudinal axis (*). Most of these unresponsive neurons (20/21) were found outside of the Psol. Only one Psol neuron was unresponsive to vestibular stimulation about the longitudinal axis. The null planes of seventeen neurons were not measured (▫). Of these neurons, 15/17 were within the Psol and were sensitive to FIG. 8. Localization of vestibularly responsive neurons within Psol. The left dorsal brain stem is illustrated in transverse sections, spaced ⬃500 m apart in A–D, left. The numbers to the right of each section indicate the distance from the caudal pole of the Psol. The area that includes Psol in each section is indicated. This area is shown at higher magnification in the right column. The locations of Psol neurons, confirmed by recovery of microlesions, are plotted in the higher magnification sections. *, location of neurons that lacked a vestibularly driven response; E, neurons whose optimal response planes corresponded to the orientation of the left anterior-right posterior semicircular canals; ●, neurons with optimal response planes corresponding to the orientation of the left posterior-right anterior semicircular canals; 䡲, neurons that responded to rotation onto both the left and right sides; ▫, neurons that were vestibularly responsive, but in which null planes were not measured. 3566 N. H. BARMACK AND V. YAKHNITSA axis. When the vestibular stimulus was stopped, the discharge of these responses returned to nonperiodic spontaneous levels. However, the vestibularly evoked discharge of ⬃5% of Psol neurons was not stationary. In these neurons, sinusoidal vestibular stimulation about the longitudinal axis evoked increased discharge when the rabbit was rotated onto the side that was ipsilateral to the recorded Psol neuron (Fig. 9A). However, when the sinusoidal vestibular stimulation was stopped, the neuron continued to oscillate at a frequency that reflected its stimulus history (Fig. 9, B and C). These slow oscillations lasted 100 –300 s. DISCUSSION Psol and its transmitter-specific circuitry In the rabbit the Psol consists of a cluster of 2,300 neurons that lies dorsolateral to the tractus solitarius in its caudal most aspect and wedged between the MVN and DVN at its most rostral aspect (Fig. 10) (Barmack et al. 1998). In this study we Comparison of the physiology of Psol neurons with the physiology of other neurons receiving projections from Psol neurons The optimal response planes for neurons in the nucleus reticularis gigantocellularis (NRGc) (Fagerson and Barmack 1995), -nucleus (Barmack et al. 1993a), dmcc (Barmack 1996), and climbing fiber responses of Purkinje cells in the uvula-nodulus (Barmack and Shojaku 1995; Fushiki and Barmack 1997), like those of Psol neurons, were consistent with stimulation of the vertical semicircular canals and utricular otoliths. In each of these structures, the optimal response planes correspond to the anatomical planes of the vertical semicircular canals or utricular otoliths. In none of the Psol, NRGc, -nucleus, dmcc, or uvula-nodulus was there any physiological evidence of activity modulated by vestibular stimulation in the plane of the horizontal semicircular canal. There are differences as well as similarities in the evoked neuronal activity in each of these locations. For example, we found that Psol neurons lacked optokinetic sensitivity. However, in the -nucleus and uvula-nodulus, many of the neurons driven by vestibular stimulation are also responsive to vertical Downloaded from http://jn.physiology.org/ by 10.220.32.246 on June 14, 2017 FIG. 9. Vestibularly induced oscillations in Psol neurons. The responses of some Psol neurons persisted after the sinusoidal stimulus was stopped. The discharge of this neuron, recorded from the right Psol, continued to oscillate after sinusoidal roll stimulation. A: the peristimulus histograms for vestibular stimulation about the longitudinal axis at 0.3 and 0.5 Hz are illustrated. B: when the vestibular stimulation stopped, the Psol neuron continued to oscillate. C: the oscillations attenuated over 1–2 min as indicated by the decreased number of action potentials in each burst measured immediately after the vestibular stimulation was stopped (0 min) and at 1 and 2 min later. have shown that Psol neurons respond to vestibular stimulation. In particular we have shown that most Psol neurons are modulated during static and dynamic roll-tilt about the longitudinal axis. Psol neurons have optimal response planes that are consistent with stimulation of either the ipsilateral anterior or posterior semicircular canals and the utricular otoliths. Psol neurons are not modulated by rotation about the vertical axis nor are they modulated by optokinetic stimulation. The identity of the Psol as the exclusive source of the vestibular GABAergic projection to both the -nucleus and the dmcc is reinforced by observations concerning the retrograde and transneuronal transport of the ␣-herpes virus following infection of the uvula-nodulus of gerbils (Kaufman et al. 1996). Forty-eight hours after a postuvula-nodulus infection, the ␣-herpes virus was found only in the dmcc and -nucleus. However, after a 50-h survival period, the virus labeled a cell group that corresponded to the location of Psol, contralateral to uvula-nodulus injection site. Of equal interest, following the 50-h survival period, other neurons within the vestibular complex were only sparsely labeled. The Psol is embedded in neuronal circuitry that provides an important source of vestibular climbing fiber inputs to the cerebellum. This transmitter-specific circuitry is illustrated in Fig. 10. Stimulation of the vertical semicircular canals or the utricular otolith activates glutamatergic primary vestibular afferents that synapse on ipsilateral Psol neurons (Raymond et al. 1984). Psol neurons are GABAergic and project to the ipsilateral NRGc and to the dmcc and -nucleus (Barmack et al. 1998). The dmcc and -nucleus, in turn, project to the contralateral cerebellar uvula-nodulus (Barmack 1996; Brodal 1976; Eisenman et al. 1983; Kaufman et al. 1996; Sato and Barmack 1985; Whitworth et al. 1983). The transmitters for this projection include aspartate or glutamate (Wiklund et al. 1982; Zhang and Ottersen 1993) and corticotropin-releasing factor (Barmack and Young 1990; Mugnaini and Nelson 1989; Young et al. 1986). The Purkinje cells of the uvula-nodulus project onto the ipsilateral Psol (N. H. Barmack, Z.-Y. Qian, and J. Yoshimura, unpublished results). PARASOLITARY VESTIBULAR PHYSIOLOGY 3567 optokinetic stimulation (Barmack and Shojaku 1995; Barmack et al. 1993b; Fushiki and Barmack 1997). Optokinetic information from the medial terminal nucleus converges directly onto neurons in the -nucleus (Giolli et al. 1984). cessation of long-term periodic vestibular stimulation (Kleinschmidt and Collewijn 1975). These vestibularly evoked oscillations may reflect a fundamental system response to periodic motion. Vestibularly evoked oscillations in Psol neurons Comparison of Psol with “classical” vestibular nuclei Some Psol neurons were driven into oscillations by repetitive sinusoidal roll stimulation. In this regard, these Psol neurons are similar to, and maybe responsible for, climbing fiber oscillations that we have previously recorded from Purkinje neurons in the uvula-nodulus (Barmack and Shojaku 1992). There are two classes of explanations that could account for these oscillations: the oscillations represent intrinsic membrane properties of certain neurons and the oscillations represent network properties of the vestibular primary afferent 3 Psol 3 -nucleus 3 uvula-nodulus 3 Psol circuit. In either case, these oscillations comprise an adaptive function that could account for the persistence of periodic reflexive eye movements that last 1–2 min after the The Psol is the one vestibular nucleus for which we can identify a clear encoding function. Psol neurons encode vestibular spatial orientation about the longitudinal axis. The characteristics of Psol neurons differ from the more “classic” vestibular neurons in several respects: 1) the Psol is composed exclusively of an homogenous group of small GABAergic, principal neurons, without evidence of inhibitory interneurons (Barmack et al. 1998). Other vestibular nuclei are composed of a mixture of non-GABAergic principal neurons and inhibitory neurons that may be GABAergic or glycinergic (Behar et al. 1994; Kumoi et al. 1987). 2) The Psol lacks a commissural projection. Other vestibular nuclei (with the exception of Deiter’s nucleus) have a com- Downloaded from http://jn.physiology.org/ by 10.220.32.246 on June 14, 2017 FIG. 10. Transmitter-specific circuitry related to the parasolitary nucleus and central vestibular function. Several transmitter-specific circuits are represented in this schematic. One projection, represented in green, expresses glutamate: 1) vestibular primary afferents 3 vestibular complex and cerebellum. Two projections express GABA (red): 1) Psol 3 -nucleus, dorsomedial cell column and nucleus reticularis gigantocellularis (NRGc) and 2) cerebellar folia 9 –10 3 Psol. One projection expresses acetylcholine (blue): medial vestibular nucleus 3 cerebellar folia 9 –10. This projection is primarily ipsilateral but also includes a contralateral component. One circuit expresses acetylcholine (blue) and GABA (red): NPH 3 dorsal cap (dc). This projection is primarily contralateral but also includes an ipsilateral component (Barmack et al. 1993a; Caffé et al. 1996; De Zeeuw et al. 1993). One projection expresses both glutamate (green) and corticotropin-releasing factor (CRF) (yellow): vestibular inferior olive (-nucleus and dorsomedial cell column) 3 contralateral cerebellum folia 9 –10. Abbreviations: Amb, nucleus ambiguus; , -nucleus; CF, climbing fiber; CRF, corticotropinreleasing factor; dc, dorsal cap of Kooy; DAO, dorsal accessory olive; dmcc, dorsomedial cell column; icp, inferior cerebellar peduncle; Fl, flocculus; Gr, granule cell; LRN, lateral reticular nucleus; MAO, medial accessory olive; MF, mossy fiber; P, Purkinje neuron; PF, parallel fiber; PFl, paraflocculus; PO, principal olive; Pyr, pyramidal tract; 12n, hypoglossal nerve. 3568 N. H. BARMACK AND V. YAKHNITSA Functional implications of Psol circuitry It is interesting to speculate about the possible function of the circuitry in which the Psol is embedded. Primary vestibular inputs, say on the left side, would activate left Psol neurons. These neurons would in turn inhibit neurons in the left -nucleus and dmcc. This would decrease the climbing fiber signals to the right uvula-nodulus. Because the modulation of the simple spikes of cerebellar Purkinje cells is antiphasic to climbing fiber signals, a decrease in climbing fiber input would cause an increase in simple spike discharge (Fushiki and Barmack 1997). This increased simple spike discharge in the right uvula-nodulus would reduce the activity of neurons in the right Psol. Thus activation of neurons in one Psol by primary vestibular afferents would lead to a reciprocal reduction in activity in the contralateral Psol mediated by: left Psol 3 left -nucleus and dmcc 3 right nodulus 3 right Psol. There is little doubt that this circuitry is critical for providing information about spatial orientation about the longitudinal axis. It remains for future experiments to determine the behavioral significance of this circuitry. Experiments that link its role to otolithic function, vestibular adaptation and possible vestibular-autonomic interactions seem most promising. We thank M. Westcott-Hodson for expert histological, technical, and artistic assistance. We thank S.-H. Park for technical and graphic assistance. The antiserum to GAD was provided by the Laboratory of Clinical Science, National Institute of Mental Health. The antiserum was developed by Dr. I. J. Kopin in association with Drs. W. Oertel, D. E. Schmechel and M. Tappaz. This research was supported by National Eye Institute Grant EY-04778. Address for reprint requests: N. H. Barmack, Neuronal Plasticity Group, L111, Oregon Health Sciences University, 3181 SW Sam Jackson Park Rd., Portland, OR 97201. Received 4 January 2000; accepted in final form 6 March 2000. REFERENCES ALLEN WF. Origin and distribution of the tractus solitarius in the guinea pig. J Comp Neurol 35: 171–204, 1923. ALTSCHULER SM, BAO X, BIEGER D, HOPKINS DA, AND MISELIS RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol 283: 248 –268, 1989. BARMACK NH. GABAergic pathways convey vestibular information to the beta nucleus and dorsomedial cell column of the inferior olive. Ann NY Acad Sci 781: 541–552, 1996. BARMACK NH. The central vestibular system. In: Neuroscience Secrets, edited by Wong-Riley MTT. Philadelphia: Hanley and Belfus, 1999, p. 115–140. BARMACK NH, BAUGHMAN RW, AND ECKENSTEIN FP. Cholinergic innervation of the cerebellum of the rat by secondary vestibular afferents. In: Sensing and Controlling Motion: Vestibular and Sensorimotor Function, edited by Cohen B, Tomko DL, and Guedry F. New York: New York Acad. Sci., 1992, p. 566 –579. BARMACK NH, FAGERSON M, AND ERRICO P. Cholinergic projection to the dorsal cap of the inferior olive of the rat, rabbit and monkey. J Comp Neurol 328: 263–281, 1993a. BARMACK NH, FAGERSON M, FREDETTE BJ, MUGNAINI E, AND SHOJAKU H. Activity of neurons in the beta nucleus of the inferior olive of the rabbit evoked by natural vestibular stimulation. Exp Brain Res 94: 203–215, 1993b. BARMACK NH, FREDETTE BJ, AND MUGNAINI E. Parasolitary nucleus: a source of GABAergic vestibular information to the inferior olive of rat and rabbit. J Comp Neurol 392: 352–372, 1998. BARMACK NH AND SHOJAKU H. Vestibularly induced slow oscillations in climbing fiber responses of Purkinje cells in the cerebellar nodulus of the rabbit. Neuroscience 50: 1–5, 1992. BARMACK NH AND SHOJAKU H. Vestibular and visual signals evoked in the uvula-nodulus of the rabbit cerebellum by natural stimulation. J Neurophysiol 74: 2573–2589, 1995. BARMACK NH AND YOUNG WSI. Optokinetic stimulation increases corticotropin-releasing factor mRNA in inferior olivary neurons of rabbits. J Neurosci 10: 631– 640, 1990. BEHAR T, MA W, HUDSON L, AND BARKER JL. Analysis of the anatomical distribution of GAD67 mRNA encoding truncated glutamic acid decarboxylase proteins in the embryonic rat brain. Dev Brain Res 77: 77– 87, 1994. BRODAL A. The olivocerebellar projection in the cat as studied with the method of retrograde axonal transport of horseradish peroxidase. II. The projection to the uvula. J Comp Neurol 166: 417– 426, 1976. BÜTTNER-ENNEVER J. Patterns of connectivity in the vestibular nuclei. In: Sensing and Controlling Motion, edited by Cohen B, Tomko DL, and Guedry F. New York: Ann. NY Acad. Sci., 1992, p. 363–378. CAFFÉ AR, HAWKINS RK, AND DE ZEEUW CI. Coexistence of choline acetyltransferase and GABA in axon terminals in the dorsal cap of the rat inferior olive. Brain Res 724: 136 –140, 1996. DE ZEEUW CI, WENTZEL P, AND MUGNAINI E. Fine structure of the dorsal cap of the inferior olive and its GABAergic and non-GABAergic input from the nucleus prepositus hypoglossi in rat and rabbit. J Comp Neurol 327: 63– 82, 1993. DEECKE L, SCHWARZ DWF, AND FREDRICKSON JM. Vestibular responses in the rhesus monkey ventroposterior thalamus. II. Vestibulo-proprioceptive convergence at thalamic neurons. Exp Brain Res 30: 219 –232, 1977. EISENMAN LM, SIEGER DD, AND BLATT GJ. The olivocerebellar projection to the uvula in the mouse. J Comp Neurol 221: 53–59, 1983. EPEMA AH, GERRITS NM, AND VOOGD J. Commissural and intrinsic connections of the vestibular nuclei in the rabbit: a retrograde labeling study. Exp Brain Res 71: 129 –146, 1988. EPEMA AH, GERRITS NM, AND VOOGD J. Secondary vestibulocerebellar projections to the flocculus and uvulo-nodular lobule of the rabbit: a study using HRP and double fluorescent tracer techniques. Exp Brain Res 80: 72– 82, 1990. FAGERSON MH AND BARMACK NH. Responses to vertical vestibular stimulation of neurons in the nucleus reticularis gigantocellularis in rabbits. J Neurophysiol 73: 2378 –2391, 1995. FERNANDEZ C AND GOLDBERG JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol 39: 970 –984, 1976. FUSHIKI H AND BARMACK NH. Topography and reciprocal activity of cerebellar Purkinje cells in the uvula-nodulus modulated by vestibular stimulation. J Neurophysiol 78: 3083–3094, 1997. GACEK RR. Location of commissural neurons in the vestibular nuclei of the cat. Exp Neurol 59: 479 – 491, 1978. Downloaded from http://jn.physiology.org/ by 10.220.32.246 on June 14, 2017 missural projection (Gacek 1978; Ito et al. 1985; Newlands et al. 1989). 3) The Psol receives afferents from other vestibular nuclei but does not project to these nuclei. The MVN, DVN, and SVN have reciprocal projections with other vestibular nuclei (Epema et al. 1988). 4) The Psol is the exclusive origin of the GABAergic vestibular projection to the inferior olive and perhaps the nucleus reticularis gigantocellularis. Other vestibular nuclei have a wider array of ascending and descending projections (Büttner-Ennever 1992; Deecke et al. 1977). 5) Psol neurons do not project as mossy fibers to the cerebellum. Rather the Psol cerebellar projection is mediated exclusively by the vestibular climbing fiber projections of the inferior olive. Other vestibular nuclei (MVN, DVN, and SVN) project bilaterally to the cerebellum as mossy fibers. At least a subset of this mossy fiber projection is cholinergic (Barmack et al. 1992; Epema et al. 1990). 6) The Psol, like other vestibular nuclei, receives a direct projection from the ipsilateral cerebellum (Barmack 1999). This cerebellar projection to the Psol and other vestibular nuclei is not uniform. Parts of each of these vestibular nuclei receive no cerebellar projection (N. H. Barmack, Z.-Y. Qian, and J. Yoshimura, unpublished data). PARASOLITARY VESTIBULAR PHYSIOLOGY RAYMOND J, NIEOULLON A, DEMEMES D, AND SANS A. Evidence for glutamate as a neurotransmitter in the cat vestibular nerve: radioautographic and biochemical studies. Exp Brain Res 56: 523–531, 1984. RUGGIERO DA, MTUI EP, OTAKE K, AND ANWAR M. Vestibular afferents to the dorsal vagal complex: substrate for vestibular-autonomic interactions in the rat. Brain Res 743: 294 –302, 1996. SATO Y AND BARMACK NH. Zonal organization of the olivocerebellar projection to the uvula in rabbits. Brain Res 359: 281–291, 1985. WALBERG F, POMPEIANO O, BRODAL A, AND JANSEN J. The fastigiovestibular projection in the cat. An experimental study with silver impregnation methods. J Comp Neurol 118: 49 –75, 1962a. WALBERG F, POMPEIANO O, WESTRUM LE, AND HAUGLIE-HANSSEN E. Fastigioreticular fibers in cat. An experimental study with silver methods. J Comp Neurol 119: 187–199, 1962b. WHITWORTH RH JR, HAINES DE, AND PATRICK GW. The inferior olive of a prosimian primate, Galago sneegalensis. II. Olivocerebellar projections to the vestibulocerebellum. J Comp Neurol 219: 228 –240, 1983. WIKLUND L, TOGGENBURGER G, AND CUENOD M. Aspartate: possible neurotransmitter in cerebellar climbing fibers. Science 216: 78 –79, 1982. YOUNG WS III, WALKER LC, POWERS RE, DESOUZA EB, AND PRICE DL. Corticotropin-releasing factor mRNA is expressed in the inferior olives of rodents and primates. Mol Brain Res 1: 189 –192, 1986. ZHANG N AND OTTERSEN OP. In search of the identity of the cerebellar climbing fiber transmitter: immunocytochemical studies in rats. Can J Neurol Sci 20 Suppl 3: S36 –S42, 1993. Downloaded from http://jn.physiology.org/ by 10.220.32.246 on June 14, 2017 GIOLLI RA, BLANKS RHI, AND TORIGOE Y. Pretectal and brain stem projections of the medial terminal nucleus of the accessory optic system of the rabbit and rat as studied by anterograde and retrograde neuronal tracing methods. J Comp Neurol 227: 228 –251, 1984. ITO JI, MATSUOKA I, SASA M, AND TAKAORI S. Commissural and ipsilateral internuclear connection of vestibular nuclear complex of the cat. Brain Res 341: 73– 81, 1985. KAUFMAN GD, MUSTARI MJ, MISELIS RR, AND PERACHIO AA. Transneuronal pathways to the vestibulocerebellum. J Comp Neurol 370: 501–523, 1996. KLEINSCHMIDT HJ AND COLLEWIJN H. A search for habituation of vestibuloocular reactions to rotatory and linear sinusoidal accelerations in the rabbit. Exp Neurol 47: 257–267, 1975. KORTE GE. The brainstem projection of the vestibular nerve in the cat. J Comp Neurol 184: 279 –292, 1979. KUMOI K, SAITO N, AND TANAKA C. Immunohistochemical localization of ␥-aminobutyric acid- and aspartate-containing neurons in the guinea pig vestibular nuclei. Brain Res 416: 22–23, 1987. MUGNAINI E AND DAHL A-L. Zincaldehyde fixation for light microscopic immunocytochemistry of nervous tissues. J Histochem Cytochem 31: 1435– 1438, 1983. MUGNAINI E AND NELSON BJ. Corticotropin-releasing factor (CRF) in the olivo-cerebellar system and feline olivary hypertrophy. In: The Olivocerebellar System in Motor Control, edited by Strata P. Berlin: SpringerVerlag, 1989, p. 187–197. NEWLANDS SD, KEVETTER GA, AND PERACHIO AA. A quantitative study of the vestibular commissures in the gerbil. Brain Res 487: 152–157, 1989. 3569